Hydroacylation
Hydroacylation is a type of organic reaction in which an alkene is inserted into the a formyl C-H bond. The product is a ketone. The reaction requires a metal catalyst. It is almost invariably practiced as an intramolecular reaction using homogeneous catalysts, often based on rhodium phosphines.
- RCHO + CH2=CHR' → RC(O)CH2CH2R'
With an alkyne in place of alkenes, the reaction produce an α,β-unsaturated ketone.[1]
Examples
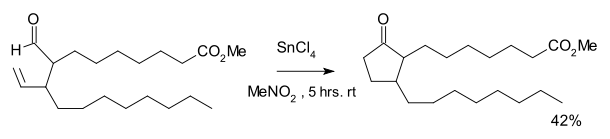
The reaction was discovered as part of a synthetic route to certain prostanoids.[2] The reaction required tin tetrachloride and a stoichiometric amount of Wilkinson's catalyst. An equal amount of a cyclopropane was formed as the result of decarbonylation.
The first catalytic application involved cycization of 4-pentenal to cyclopentanone using with Wilkinson's catalyst.[3] In this reaction the solvent was saturated with ethylene.
- CH2=CHCH2CH2CHO → (CH2)4CO
Reaction mechanism
Labeling studies establish the following regiochemistry:
- RCDO + CH2=CHR' → RC(O)CH2CHDR'
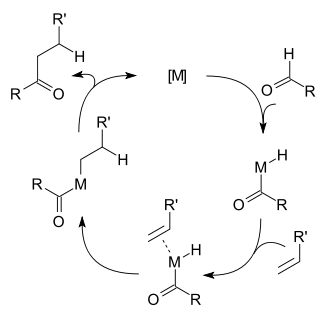
In terms of the reaction mechanism, hydroacylation begins with oxidative addition of the aldehydic carbon-hydrogen bond. The resulting acyl hydride complex next binds the alkene. The sequence of oxidative addition and alkene coordination is often unclear. Via migratory insertion, the alkene inserts into either the metal-acyl or the metal-hydride bonds. In the final step, the resulting alkyl-acyl or beta-ketoalkyl-hydride complex undergoes reductive elimination.[1] A competing side-reaction is decarbonylation of the aldehyde. This process also proceeds via the intermediacy of the acyl metal hydride:
- R"C(O)-MLn-H → R"-M(CO)Ln-H
This step can be followed by reductive elimination of the alkane:
- R"-M(CO)Ln-H → R"-H + M(CO)Ln
Asymmetric hydroacylation
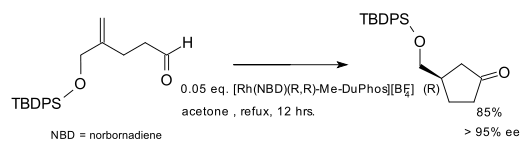
Hydroacylation as an asymmetric reaction was demonstrated in the form of a kinetic resolution.[4][5] A true asymmetric synthesis was also described.[6][7] Both conversions employed rhodium catalysts and a chiral diphosphine ligand. In one application the ligand is Me-DuPhos:[8]
References
- Michael C. Willis (2009). "Transition Metal Catalyzed Alkene and Alkyne Hydroacylation". Chem. Rev. 110: 725–748. doi:10.1021/cr900096x.
- K. Sakai; J. Ide; O. Oda; N. Nakamura (1972). "Synthetic studies on prostanoids 1 synthesis of methyl 9-oxoprostanoate". Tetrahedron Letters. 13: 1287–1290. doi:10.1016/S0040-4039(01)84569-X.
- Transition-Metal-Promoted Aldehyde-Alkene Addition Reactions Charles F. Lochow, Roy G. Miller J. Am. Chem. Soc., 1976, 98 (5), pp 1281–1283 doi:10.1021/ja00421a050
- The Asymmetric cyclisation of substituted pent-4-enals by a chiral rhodium phosphine catalyst Brian R. James and Charles G. Young J. Chem. Soc., Chem. Commun., 1983, 1215 - 1216, doi:10.1039/C39830001215
- Catalytic decarbonylation, hydroacylation, and resolution of racemic pent-4-enals using chiral bis(di-tertiary-phosphine) complexes of rhodium(I) Brian R. James, and Charles G. Young Journal of Organometallic Chemistry Volume 285, 1985, Pages 321-332 doi:10.1016/0022-328X(85)87377-0
- Asymmetric cyclization reactions by Rh(I) with chiral ligands Yukari Tauraa, Masakazu Tanakaa, Kazuhisa Funakoshia and Kiyoshi Sakai. Tetrahedron Letters. Volume 30, Issue 46, 1989, Pages 6349-6352 doi:10.1016/S0040-4039(01)93891-2
- Asymmetric cyclization reactions. Cyclization of substituted 4-pentenals into cyclopentanone derivatives by rhodium(I) with chiral ligands Yukari Taura, Masakazu Tanaka, Xiao-Ming Wu, Kazuhisa Funakoshi and Kiyoshi Sakai. Tetrahedron. Volume 47, Issue 27, 1991, Pages 4879-4888 doi:10.1016/S0040-4020(01)80954-6
- Synthesis of D- and L-Carbocyclic Nucleosides via Rhodium-Catalyzed Asymmetric Hydroacylation as the Key Step Patricia Marce, Yolanda Dıaz, M. Isabel Matheu, Sergio Castillon Org. Lett., 2008, 10 (21), pp 4735–4738 doi:10.1021/ol801791g