Prostanoid
Prostanoids are a subclass of eicosanoids consisting of the prostaglandins (mediators of inflammatory and anaphylactic reactions), the thromboxanes (mediators of vasoconstriction), and the prostacyclins (active in the resolution phase of inflammation.)
Biosynthesis
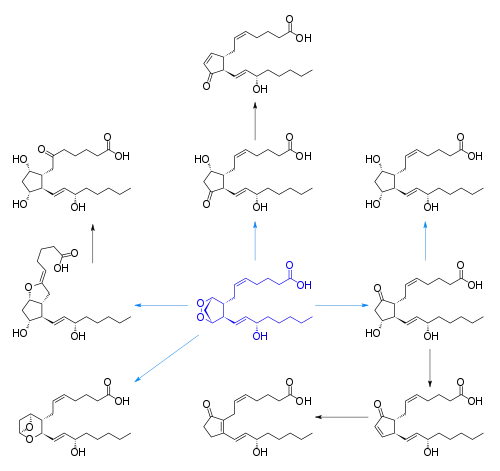
Cyclooxygenase (COX) catalyzes the conversion of the free essential fatty acids to prostanoids by a two-step process. In the first step, two molecules of O2 are added as two peroxide linkages and a 5-member carbon ring is forged near the middle of the fatty acid chain. This forms the short-lived, unstable intermediate Prostaglandin G (PGG). One of the peroxide linkages sheds a single oxygen, forming PGH. (See diagrams and more detail at Cyclooxygenase). All other prostanoids originate from PGH (as PGH1, PGH2, or PGH3).
The image at right shows how PGH2 (derived from Arachidonic acid) is converted:
- By PGE synthetase into PGE2 (which in turn is converted into PGF2)
- By PGD synthetase into PGD2
- By Prostacyclin synthase into prostacyclin (PGI2)
- By Thromboxane synthase into thromboxanes
The three classes of prostanoids have distinctive rings in the center of the molecule. They differ in their structures. The PGH compounds (parents to all the rest) have a 5-carbon ring, bridged by two oxygens (a peroxide.) The derived prostaglandins contain a single, unsaturated 5-carbon ring. In prostacyclins, this ring is conjoined to another oxygen-containing ring. In thromboxanes the ring becomes a 6-member ring with one oxygen.
Production of PGE2 in bacterial and viral infections appear to be stimulated by certain cytokines, e.g., interleukin-1.[1]
See also
References
- University of Kansas Medical Center (2004). "Eicosanoids and Inflammation" (PDF). Archived from the original (PDF) on 2005-05-16. Retrieved 2007-01-05.