Isomaltulose
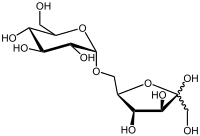
Isomaltulose is a disaccharide carbohydrate composed of glucose and fructose. The glucose and fructose are linked by an alpha-1,6-glycosidic bond (chemical name: 6-0-α-D-glucopyranosyl-D-fructose). Isomaltulose is present in honey[1] and sugarcane extracts.[2] It tastes similar to sucrose (table sugar) with half the sweetness. Isomaltulose, also known by the trade name Palatinose, is manufactured by enzymatic rearrangement (isomerization) of sucrose from beet sugar. The enzyme and its source were discovered in Germany in 1950,[3] and since then its physiological role and physical properties have been studied extensively.[4][5][6] Isomaltulose has been used as an alternative to sugar in foods in Japan since 1985, in the EU since 2005, in the US since 2006, and in Australia and New Zealand since 2007,[7] besides other countries worldwide. Analytical methods for characterization and assay of commercial isomaltulose are laid down, for example, in the Food Chemicals Codex.[8] Its physical properties closely resemble those of sucrose, making it easy to use in existing recipes and processes.
 | |
| Names | |
|---|---|
| IUPAC name
6-O-α-D-Glucopyranosyl-D-fructose | |
| Other names
Palatinose | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.878 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H22O11 | |
| Molar mass | 342.297 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Isomaltulose is hydrogenated to produce isomalt, a minimally digestible carbohydrate that is used as a sugar replacer, for example in sugar-free candies and confectionery.
Like sucrose, isomaltulose can be digested to glucose and fructose. However, while in sucrose the glucose is linked to the anomeric carbon of the fructose (an α-1,2 linkage), in isomaltulose the linkage is to the 6 carbon (α-1,6), making isomaltulose a reducing sugar, unlike sucrose. The fructose in isomaltulose exists in a ring structure that readily opens to exhibit a carbonyl group as in ketones and aldehydes, which explains why isomaltulose is a reducing sugar.[9] In comparison with sucrose and most other carbohydrates, isomaltulose is not a significant substrate for oral bacteria. Consequently, acid production from isomaltulose in the mouth is too slow to promote tooth decay.[4]
Function
In nutrition, isomaltulose is a source of food energy, providing the same amount of energy as sucrose. Like sucrose, isomaltulose provides sweetness to foods, but isomaltulose is only about half as sweet as sucrose.[5] In food preparation and processing, both isomaltulose and sucrose have similar characteristics allowing recipes that use sucrose able to use isomaltulose instead or together.[5]
Available carbohydrate
Isomaltulose is an available carbohydrate[4] like sucrose and most other sugars or maltodextrins, in the sense that it is fully metabolised in the small intestine, and does not enter the large intestine or get excreted in urine.[10]
When eaten by humans, isomaltulose is digested completely and absorbed.[11] Its intestinal digestion involves the enzyme isomaltase, which is located at the surface of the brush border lining the inner wall of the small intestine. This enzyme is otherwise involved in the digestion of α-1,6 linkages present in starch. The products of isomaltulose digestion are glucose and fructose, which are absorbed and enter the bloodstream. Once absorbed, the glucose and fructose follow the same metabolic pathways through the body as if they were derived from sucrose.[5] While fructose is mostly converted to glucose or glycogen stores in the liver, glucose from the small intestine and liver is distributed via the circulatory system to different parts of the body where it serves cellular metabolism as an energy source directly or indirectly after storage as glycogen in the tissues of the body, especially in skeletal muscle.
Source of energy
As an available carbohydrate,[11][12] the food energy value of isomaltulose is identical to that of sucrose. For both, it is 4 kcal/g (17 kJ/g), a value that is used in food labelling or dietary planning.
Slow and sustained release of carbohydrate and energy
Isomaltulose is slow to be digested and absorbed, and is therefore gradually released as glucose and fructose into the bloodstream. After ingestion, the enzymatic digestion of sucrose and isomaltulose occur on the same sucrase-isomaltase enzyme complex, which is located in the small intestine.[10][13] Several studies show that this complex breaks down isomaltulose more slowly than sucrose. The maximum rate at which isomaltase can process isomaltulose (Vmax) is 4.5 times lower than that of sucrase for sucrose.[14]
As a result of its slow digestion, isomaltulose travels further through the human small intestine than does sucrose, as evidenced by the difference in incretin responses they elicit. The incretin hormone glucose-dependent insulinotropic polypeptide (GIP) is secreted from the earlier (proximal) part of the small intestine in lower amounts after ingestion of isomaltulose than sucrose, whereas the incretin hormone glucagon-like peptide-1 (GLP-1) is secreted from a later (distal) part of the small intestine in higher amounts with isomaltulose than with sucrose.[15][16]
Compared with sucrose, the absorption of energy as carbohydrate from isomaltulose is prolonged.[16] The resulting sustained energy supply to the body from isomaltulose is reflected in the prolonged shape of the blood glucose concentration response curve.[6]
Lower blood glucose and insulin response
The blood glucose and insulin concentrations after ingestion of isomaltulose are lower than those due to sucrose or glucose, giving isomaltulose a glycemic index (GI) of 32 as recorded in the Sydney University GI database,[17] compared to 67 for sucrose and 100 for glucose, making isomaltulose a particularly low-GI carbohydrate (GI<55).
Confirmation of a low glycaemic response to isomaltulose is provided in numerous studies for different population groups including healthy people, overweight or obese persons, prediabetic persons, and type 1 or type 2 diabetes patients.[12][18][19][20][15][16][21][22] Among these studies, all show the lower blood glucose response of isomaltulose and where tested also show the associated reduction in the blood insulin response. A significant role for the incretin hormone GLP-1 has been established, which is secreted in response to distal carbohydrate absorption and limits the rise in blood glucose concentration after a meal.[15][16][22]
A claim corresponding to the low glycemic response of isomaltulose and its potential to lower the blood glucose response to foods when replacing other sugars has been approved in EU legislation[23] following the publication of a positive opinion from the European Food Safety Authority.[24]
In the long term, when eating a diet including carbohydrate, avoiding undesirably high concentrations of glucose in blood and the associated demand for insulin, is supportive of the prevention and management of diabetes mellitus, cardiovascular disease, and possibly overweight and obesity—as indicated by the International Carbohydrate Quality Consortium consensus of expert nutrition scientists.[25] Continuous monitoring of 24-h blood glucose concentration following diets including isomaltulose instead of sucrose lowers the blood glucose profile over the day, as a result of a lower blood glucose response to individual meals.[26]
A lower glycemic diet can be achieved by choosing foods with low or reduced glycemic properties, more specifically by choosing lower GI foods from within each food group (fruit, vegetable, whole grains, etc.). The use of Isomaltulose in place of sucrose and other carbohydrates allows for the production of foods with reduced GI. Several studies provide evidence of improvements in both blood glucose control and lipid metabolism in both diabetic and non-diabetic persons upon regular consumption of isomaltulose when compared with other carbohydrates such as sucrose, maltodextrin, or glucose.[19][27][28][29][30][31][32]
Effect on fat oxidation
Compared to other carbohydrates, isomaltulose ingestion is associated with higher rates of fat oxidation and lower rates of fat storage. Mechanistically this involves a lower blood glucose concentration, which then provides a reduced stimulus to insulin secretion, which in turn allows more fatty acids to be released from adipose tissue for oxidation as an energy source. The lower insulin concentration also decreases carbohydrate oxidation, allowing more fatty acids to be oxidized. A lower insulin concentration also lowers the rate of liver free fatty acid recycling via plasma triglycerides and reduces the storage of triglycerides in adipose tissue. Practical implications include higher rates of fat oxidation after ingestion of isomaltulose than higher glycaemic carbohydrates. This has been shown in many studies with different areas of focus:
Weight management and body composition
Studies have looked at the effects on fat oxidation and other metabolic responses when replacing sugars with isomaltulose in meals (or drinks) taken by healthy or overweight to obese adults, with or without impaired glucose tolerance, while largely sedentary.[20][33][34][35][36] These studies have shown isomaltulose to have a role in reducing adiposity, at least central obesity. Abdominal fat decreases when consuming isomaltulose instead of sucrose (sugar replacement) or instead of breakfast calories (largely carbohydrate replacement).[19][27][28] This is brought about at least in part by a lower GIP and higher GLP-1 response when carbohydrate is slow to digest and is absorbed slowly in the lower (distal) small intestine.[37]
Physical activity and sports nutrition
Others studies have examined the potential benefits of slow and sustained release of carbohydrate during physical activity. Using isomaltulose in place of other ingested carbohydrates, higher rates of fat oxidation also occur during endurance activities, where preserving glycogen is important.[34][38][39] In addition, trials using a recovery protein drink have shown that incorporating isomaltulose and a nutritional supplement (β-hydroxy- β-methylbutyrate ) may help recovery from resistance exercise—so reducing of muscle damage and improving athletic performance.[40]
Type 1 diabetes patients engaging in physical activity
In people with type 1 diabetes, taking isomaltulose instead of glucose during moderate carbohydrate loading before exercise improves blood glucose control and protects against hypoglycemia while maintaining running performance.[41] The reduced risk of exercise-induced hypoglycemia arises in part from a lower requirement for insulin by injection (50% lower) when using isomaltulose and in part from the higher contribution of fat oxidation to energy metabolism, which preserves glycogen stores, further reducing the risk of hypoglycemia.
Cognitive performance (mood and memory)
The rate of glucose supply from dietary carbohydrates can affect cognitive performance, with effects on mood and memory having been shown in several studies that compared isomaltulose with higher glycaemic carbohydrates taken at breakfast, showing improvements in mood and memory in healthy children, middle-aged adults, and aged adults.[42][43][44][45]
Oral health
Isomaltulose is ‘kind to teeth’. Fermentation of carbohydrates by bacteria in the mouth (especially on the teeth) is responsible for the formation of dental plaque and oral acids. The acid initiates tooth demineralisation and tooth decay (dental caries). Isomaltulose largely resists fermentation by oral bacteria and is the first carbohydrate of its kind with negligible acid production on teeth, as shown by pH telemetry. The evidence is strong and provides the basis for ‘kind to teeth’ claims approved by both the Food and Drug Administration in the USA [46][47] and European authorities following a positive opinion from the European Food Safety Authority.[24]
Use
Isomaltulose is used in foods, drinks and health products owing to several of its properties. It is used in foods and beverages, where it provides a natural sucrose-like sweetness profile with a sweetening power about half that of sucrose, and no aftertaste.[4] It has very low moisture absorption (hygroscopy), giving it free-flowing properties in instant powders, which because of their low risk of lumping can easily be used in drinks and other instant products. It is highly stable during processing, including acidic conditions and environments where bacteria might grow. In sports beverages, for instance, isotonicity (osmotic pressure equal to that of fluids in the body) can be maintained during storage over the beverage's shelf-life.
Isomaltulose finds application in baked goods, pastry glazings and icings, breakfast cereals, cereal bars, dairy produce, sugar confectionery (e.g. chocolates, jellies, chewy confections and chewing or bubble gum), frozen desserts, fruit-juice beverages, malt beverages, sports beverages, energy drinks, instant drinks, and special and clinical nutrition feeds.[4][48]
Isomaltulose in permitted for use in foods and drinks in many regions worldwide. For example, it is generally recognized as safe (GRAS) by the U.S. Food and Drug Administration,[48] is approved as a novel food by the European Commission,[49] and in Japan has the status FOSHU (food for specific health use).[50]
External links
- Webpages dedicated to isomaltulose
- Marketing of isomaltulose as a novel food or novel food ingredient in the European Union
- Assessment of the glycaemic index of isomaltulose
- Oral health claims with isomaltulose in the USA
- Article on innovative low-glycaemic carbohydrates
- Webpages further describing Isomaltulose (Palatinose)
References
- Siddiqua, I.R; Furgala, B (1967). "Isolation and characterization of oligosaccharides from honey". Journal of Apicultural Research. 6 (3): 139–145. doi:10.1080/00218839.1967.11100174.
- Egglestone, G; Grisham, M (2003). "Oligosaccharides in cane and their formation on cane deterioration". ACS Symposium Series. 849 (16): 211–232. doi:10.1021/bk-2003-0849.ch016.
- Weidenhagen, R; Lorenzo, A.D (1957). "Palatinose (6-0-alpha-D-glucopyranosyl-D-fructofuranose), ein neues bakterielles Umwandlungsprodukt der Saccharose [Palatinose (6-0-alpha-D-glucopyranosyl-D-fructofuranose), a new bacterial conversion of sucrose product]". Zeitschrift für die Zuckeridustrie. 7: 533–534.
- Sentko, A. and Willibald-Ettle, I. (2012). "Isomaltulose." In: Sweeteners and Sugar Alternatives in Food Technology, 2nd Ed. Editors O'Donnell, K. & Kearsley, M.W. Wiley-Blackwell. Oxford, UK. ISBN 978-0-470-65968-7
- Lina, B.A.R.; Jonker, D.; Kozianowski, G. (2002). "Isomaltulose (Palatinose): A review of biological and toxicological studies". Food and Chemical Toxicology. 40 (10): 1375–81. doi:10.1016/S0278-6915(02)00105-9. PMID 12387299.
- Maresch, C.C; Petry, S.F; Theis, S; Bosy-Westphal, A; Linn, T (2017). "Low Glycemic Index Prototype Isomaltulose-Update of Clinical Trials". Nutrients. 9 (4): 1–12. doi:10.3390/nu9040381. PMC 5409720. PMID 28406437.
- ANZFA (2007). "Australia New Zealand Food Standards Code – Amendment No. 92 – 2007" (PDF). Commonwealth of Australia Gazette (FSC 34 Thursday, 2 August 2007). Retrieved 9 February 2018.
- Food Chemical Codex (2010). Monograph on Isomaltulose (7th ed.). Rockville, MD 20852-1790: US Pharmacopeial Convention. pp. 546–548. ISBN 9781889788852. Archived from the original on 2015-12-04. Retrieved 2015-07-22.CS1 maint: location (link)
- O'Donnell, Kay; Kearsley, Malcolm (2012-07-13). Sweeteners and Sugar Alternatives in Food Technology. John Wiley & Sons. ISBN 9781118373972.
- Livesey, G (2014). Carbohydrate Digestion, Absorption, and Fiber. Reference Module in Biomedical Sciences. doi:10.1016/B978-0-12-801238-3.00043-X. ISBN 9780128012383.
- Holub, I; Gostner, A; Theis, S; Nosek, L; Kudlich, T; Melcher, R; Scheppach, W. (2010). "Novel findings on the metabolic effects of the low glycaemic carbohydrate isomaltulose (Palatinose)". British Journal of Nutrition. 103 (12): 1730–7. doi:10.1017/S0007114509993874. PMC 2943747. PMID 20211041.
- Macdonald, I; Daniel, J (1983). "The bioavailability of isomaltulose in man and rat". Nutrition Reports International. 28 (5): 1083–1090.
- Dahlqvist, A; Auricchio, S; Semenza, G; Prader, A (1963). "Human intestinal disaccharidases and hereditary disaccharide intolerance". Journal of Clinical Investigation. 42 (4): 556–562. doi:10.1172/JCI104744. PMC 289315. PMID 14024642.
- Sentko, A; Bernard, J (2011). Isomaltulose In: Alternative Sweeteners. Ed: L. O'Brien Nabors (4th ed.). Boca Raton, London, New York: CRC Press, Taylor & Francis Group. pp. 423–438. ISBN 978-1-4398-4614-8. e-book ISBN 978-1-4398-4615-5
- Maeda, A; Miyagawa, J; Miuchi, M; Nagai, E; Konishi, K; Matsuo, T; Tokuda, M; Kusunoki, Y; Ochi, H; Murai, K; Katsuno, T; Hamaguchi, T; Harano, Y; Namba, M (2013). "Effects of the naturally-occurring disaccharides, palatinose and sucrose, on incretin secretion in healthy non-obese subjects". Journal of Diabetes Investigation. 4 (3): 281–286. doi:10.1111/jdi.12045. PMC 4015665. PMID 24843667.
- Ang, M; Linn, T (2014). "Comparison of the effects of slowly and rapidly absorbed carbohydrates on postprandial glucose metabolism in type 2 diabetes mellitus patients: a randomized trial". American Journal of Clinical Nutrition. 100 (4): 1059–1068. doi:10.3945/ajcn.113.076638. PMID 25030779.
- "Glycaemic Index Research Service". www.glycemicindex.com. Sydney University. Retrieved 2020-07-09.
- Kawai, K; Okuda, Y; Yamashita, K (1983). "Changes in blood glucose and insulin after an oral palatinose administration in normal subjects". Endocrinologia Japonica. 32 (6): 933–936. doi:10.1507/endocrj1954.32.933. PMID 3914416.
- Yamori, Y; Mori, M; Mori, H; Kashimura, J; Sakuma, T; Ishikawa, P.M; Moriguchi, E; Moriguchi, Y (2007). "Japanese perspective for lifestyle disease risk reduction in immigrant Japanese Brazilians—A double-blind placebo-controlled intervention study on palatinose". Clinical and Experimental Pharmacology and Physiology. 34: S5–S7. doi:10.1111/j.1440-1681.2007.04759.x.
- >van Can, J.G; Ijzerman, T.H; van Loon, L.J; Brouns, F; Blaak, E.E (2009). "Reduced glycaemic and insulinaemic responses following isomaltulose ingestion: implications for postprandial substrate use". British Journal of Nutrition. 102 (10): 1408–1413. doi:10.1017/S0007114509990687. PMID 19671200.
- König, D; Theis, S; Kozianowski, G; Berg, A (2012). "Postprandial substrate use in overweight subjects with the metabolic syndrome after isomaltulose (Palatinose) ingestion". Nutrition. 26 (6): 651–656. doi:10.1016/j.nut.2011.09.019. PMID 22264450.
- Keyhani-Nejad, F; Kemper, M; Schueler, R; Pivovarova, O; Rudovich, N; Pfeiffer, A.F (2016). "Effects of Palatinose and Sucrose Intake on Glucose Metabolism and Incretin Secretion in Subjects With Type 2 Diabetes" (PDF). Diabetes Care. 39 (3): e38–e39. doi:10.2337/dc15-1891. PMID 26721819. Retrieved 28 January 2018.
- European Union (EU) Legislation, Annex. "Commission Regulation of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children's development and health". Retrieved 12 July 2015.
- "Scientific Opinion on the substantiation of health claims related to the sugar replacers xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D‐tagatose, isomaltulose, sucralose and polydextrose and maintenance of tooth mineralisation by decreasing tooth demineralisation (ID 463, 464, 563, 618, 647, 1182, 1591, 2907, 2921, 4300), and reduction of post‐prandial glycaemic responses (ID 617, 619, 669, 1590, 1762, 2903, 2908, 2920) pursuant to Article 13(1) of Regulation (EC) No 1924/2006". EFSA Journal. 9 (4): 2076. 2011. doi:10.2903/j.efsa.2011.2076.
- Augustin, L.S.A; Kendall, C.W.C; Jenkins, D.J.A; Willett, W.C; Astrup, A; Barclay, A.W; Björck, I; Brand-Miller, J.C; Brighenti, F; Buyken, A.E; Ceriello, A; La Vecchia, C; Livesey, G; Liu, S; Riccardi, G; Rizkalla, S.W; Sievenpiper, J.L; Trichopoulou, T; Wolever, T.M.S; Baer-Sinnott, S; Poli, A (2014). "Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC)". Nutrition, Metabolism and Cardiovascular Diseases. 25 (9): 795–815. doi:10.1016/j.numecd.2015.05.005. PMID 26160327.
- Henry, C.J; Kaur, B; Quek, R.Y.C; Camps, S.G (2017). "A Low Glycaemic Index Diet Incorporating Isomaltulose Is Associated with Lower Glycaemic Response and Variability, and Promotes Fat Oxidation in Asians". Nutrients. 9 (5): 473. doi:10.3390/nu9050473. PMC 5452203. PMID 28486426.
- Oizumi, T; Daimon, D; Jimbu, Y; Kameda, W; Arawaka, N; Yamaguchi, H; Ohnuma, H; Sasaki, H; Kato, T (2007). "A palatinose-based balanced formula improves glucose tolerance, serum free fatty acid levels and body fat composition". Tohoku Journal of Experimental Medicine. 212 (2): 91–99. doi:10.1620/tjem.212.91. PMID 17548953.
- Okuno, M; Kim, M.K; Mizu, M; Mori, M; Mori, H; Yamori, Y (2010). "Palatinose-blended sugar compared with sucrose: different effects on insulin sensitivity after 12 weeks supplementation in sedentary adults". International Journal of Food Science and Technology. 61 (6): 643–651. doi:10.3109/09637481003694576. PMID 20367218.
- Sakuma, M; Arai, H; Mizuno, A; Fukaya, M; Matsuura, M; Sasaki, H; Yamanaka-Okumura, H; Yamamoto, H; Taketani, Y; Doi, T; Takeda, E (2009). "Improvement of glucose metabolism in patients with impaired glucose tolerance or diabetes by long-term administration of a palatinose-based liquid formula as a part of breakfast". Journal of Clinical Biochemistry and Nutrition. 45 (2): 155–162. doi:10.3164/jcbn.09-08. PMC 2735627. PMID 19794923.
- Brunner, S; Holub, I; Theis, S; Gostner, A; Melcher, R; Wolf, P; Amann-Gassner, U; Scheppach, W; Hauner, H (2012). "Metabolic effects of replacing sucrose by isomaltulose in subjects with type 2 diabetes: a randomized double-blind trial". Diabetes Care. 35 (6): 1249–1251. doi:10.2337/dc11-1485. PMC 3357231. PMID 22492584.
- Fujiwara, T; Naomoto, Y; Motoki, T; Shigemitsu, K; Shirakawa, Y; Yamatsuji, T; Kataoka, M; Haisa, M; Fujiwara, T; Egi, M; Morimatsu, H; Hanazaki, M; Katayama, H; Morita, K; Mizumoto, K; Asou, T; Arima, H; Sasaki, H; Matsuura, M; Gunduz, M; Tanaka, N (2007). "Effects of a novel palatinose based enteral formula (MHN-01) carbohydrate-adjusted fluid diet in improving the metabolism of carbohydrates and lipids in patients with esophageal cancer complicated by diabetes mellitus". Journal of Surgical Research. 138 (2): 231–240. doi:10.1016/j.jss.2006.06.025. PMID 17254607.
- Keller, J; Kahlhöfer, J; Peter, A; Bosy-Westphal, A (2016). "Effects of Low versus High Glycemic Index Sugar-Sweetened Beverages on Postprandial Vasodilatation and Inactivity-Induced Impairment of Glucose Metabolism in Healthy Men". Nutrients. 8 (12): 1–14. doi:10.3390/nu8120802. PMC 5188457. PMID 27973411.
- Arai, H; Mizuno, A; Sakuma, M; Fukaya, M; Matsuo, K; Muto, K; Sasaki, H; Matsuura, M; Okumura, H; Yamamoto, H; Taketani, Y; Doi, T; Takeda, E (2007). "Effects of a palatinose-based liquid diet (Inslow) on glycemic control and the second-meal effect in healthy men". Metabolism. 56 (1): 115–121. doi:10.1016/j.metabol.2006.09.005. PMID 17161233.
- König, D; Luther, W; Polland, V; Theis, S; Kozianowski, G; Berg, A (2007). "Metabolic effects of low-glycemic Palatinose during long-lasting endurance exercise". Annals of Nutrition and Metabolism. 51 (Supp 1): 61.
- van Can, J.G; van Loon, L.J; Brouns, F; Blaak, E.E (2012). "Reduced glycaemic and insulinaemic responses following trehalose and isomaltulose ingestion: implications for postprandial substrate use in impaired glucose-tolerant subjects". British Journal of Nutrition. 108 (7): 1210–1217. doi:10.1017/S0007114511006714. PMID 22172468.
- Kahlhöfer, J; Karschin, J; Silberhorn-Bühler, H; Breusing, N; Bosy-Westphal, A; Kahlhofer, J; Silberhorn-Buhler, H (2016). "Effect of low glycemic-sugar-sweetened beverages on glucose metabolism and macronutrient oxidation in healthy men". International Journal of Obesity. 40 (6): 990–997. doi:10.1038/ijo.2016.25. PMID 26869244.
- Pfeiffer, A.F.H; Keyhani-Nejad, F (2018). "High Glycaemic Index Metabolic Damage—a Pivotal Role of GIP and GLP-1". Trends in Endocrinology and Metabolism. 29 (5): 289–298. doi:10.1016/j.tem.2018.03.003. PMID 29602522.
- Achten, J; Jentjens, R.L; Brouns, F; Jeukendrup, A.E (2007). "Exogenous oxidation of isomaltulose is lower than that of sucrose during exercise in men". Journal of Nutrition. 137 (5): 1143–1148. doi:10.1093/jn/137.5.1143. PMID 17449572.
- König, D; Zdzieblik, D; Holz, A; Theis, S; Gollhofer, A (2016). "Substrate Utilization and Cycling Performance Following Palatinose™ Ingestion: A Randomized, Double-Blind, Controlled Trial". Nutrients. 8 (7): 990–997. doi:10.3390/nu8070390. PMC 4963866. PMID 27347996.
- Kraemer, W.J; Hooper, D.R; Szivak, T.K; Kupchak, B.R; Dunn-Lewis, C; Comstock, B.A; Flanagan, S.D; Looney, D.P; Sterczala, A.J; DuPont, W.H; Pryor, J.L; Luk, H.Y; Maladoungdock, J; McDermott, D; Volek, J.S; Maresh, C.M (2015). "The Addition of Beta-hydroxy-beta-methylbutyrate and Isomaltulose to Whey Protein Improves Recovery from Highly Demanding Resistance Exercise". Journal of the American College of Nutrition. 34 (2): 91–99. doi:10.1080/07315724.2014.938790. PMID 25758255.
- Bracken, R.M; Page, R; Gray, B; Kilduff, L.P; West, D.J; Stephens, J.W; Bain, S.C (2012). "Isomaltulose improves glycemia and maintains run performance in type 1 diabetes". Medicine and Science in Sports and Exercise. 44 (5): 800–808. doi:10.1249/MSS.0b013e31823f6557. PMID 22051571.
- Taib, M.N; Shariff, Z.M; Wesnes, K.A; Saad, H.A; Sariman, S (2012). "The effect of high lactose-isomaltulose on cognitive performance of young children. A double blind cross-over design study" (PDF). Appetite. 58 (1): 81–87. doi:10.1016/j.appet.2011.09.004. PMID 21986189.
- Sekartini, R; Wiguna, T; Bardosono, S; Novita, D; Arsianti, T; Calame, W; Schaafsma, A (2013). "The effect of lactose-isomaltulose-containing growing-up milks on cognitive performance of Indonesian children: a cross-over study". British Journal of Nutrition. 110 (6): 1089–1097. doi:10.1017/S0007114513000135. PMID 23680182.
- Young, H; Benton, D (2014). "The effect of using isomaltulose (Palatinose) to modulate the glycaemic properties of breakfast on the cognitive performance of children". European Journal of Nutrition. 54 (6): 1013–1020. doi:10.1007/s00394-014-0779-8. PMC 4540784. PMID 25311061.
- Young, H; Benton, D (2014). "The glycemic load of meals, cognition and mood in middle and older aged adults with differences in glucose tolerance: A randomized trial". E_SPEN Journal. 9 (4): e147–e154. doi:10.1016/j.clnme.2014.04.003.
- Food and Drug Administration. "Health claims, dietary non-cariogenic carbohydrate sweeteners and dental caries". Electronic Code of Federal Regulations 21 ECFR Part 101.80. Retrieved 26 August 2015.
- Food and Drug Administration - Department of Health and Human Services, 2015. "Food labeling: Health claims; dietary noncariogenic carbohydrate sweeteners and dental caries". Code of Federal Regulations 21 CFR Part 101.80. Retrieved 26 August 2015.CS1 maint: numeric names: authors list (link)
- Food and Drug Administration, 2006. "Agency Response Letter GRAS Notice No. GRN 000184". Retrieved 14 July 2015.CS1 maint: numeric names: authors list (link)
- European Commission Decision, 25 July 2005. "Authorising the placing on the market of isomaltulose as a novel food or novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council (2005/581/EC)". Retrieved 14 June 2015.
- Japanese Ministry of Health, Labour and Welfare. "Food for Specified Health Uses (FOSHU)". Retrieved 15 July 2015.