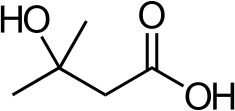
beta-Hydroxy beta-methylbutyric acid
β-Hydroxy β-methylbutyric acid[note 1] (HMB), otherwise known as its conjugate base, β-hydroxy β-methylbutyrate, is a naturally produced substance in humans that is used as a dietary supplement and as an ingredient in certain medical foods that are intended to promote wound healing and provide nutritional support for people with muscle wasting due to cancer or HIV/AIDS.[sources 1] In healthy adults, supplementation with HMB has been shown to increase exercise-induced gains in muscle size, muscle strength, and lean body mass, reduce skeletal muscle damage from exercise, improve aerobic exercise performance, and expedite recovery from exercise.[sources 2] Medical reviews and meta-analyses indicate that HMB supplementation also helps to preserve or increase lean body mass and muscle strength in individuals experiencing age-related muscle loss.[note 2][11][12][13] HMB produces these effects in part by stimulating the production of proteins and inhibiting the breakdown of proteins in muscle tissue.[11][14][15] No adverse effects from long-term use as a dietary supplement in adults have been found.[16][17][18]
 | |
 Top: β-Hydroxy β-methylbutyric acid Bottom: β-Hydroxy β-methylbutyrate | |
| Clinical data | |
|---|---|
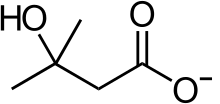
| Other names | Conjugate acid form: β-hydroxyisovaleric acid 3-hydroxyisovaleric acid Conjugate base form: hydroxymethylbutyrate |
| Routes of administration | By mouth[1] or nasogastric[2] |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolites | HMB-CoA, HMG-CoA, mevalonate, cholesterol, acetyl-CoA, acetoacetate, β-hydroxybutyrate |
| Onset of action | HMB-FA: 30–60 minutes[1] HMB-Ca: 1–2 hours[1] |
| Elimination half-life | HMB-FA: 3 hours[1] HMB-Ca: 2.5 hours[1] |
| Excretion | Renal (10–40% excreted)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.128.078 |
| Chemical and physical data | |
| Formula | C5H10O3 |
| Molar mass | 118.132 g·mol−1 |
| 3D model (JSmol) | |
| Density | ~1.1 g/cm3 at 20 °C[4] |
| Melting point | −80 °C (−112 °F) (glass)[5] |
| Boiling point | 128 °C (262 °F) at 7 mmHg[4][6] |
| |
| |
| (verify) | |
HMB is sold as a dietary supplement at a cost of about US$30–50 per month when taking 3 grams per day.[16][19][20] HMB is also contained in several nutritional products, including certain formulations of Ensure, Juven, and Myoplex.[8][21] HMB is also present in insignificant quantities in certain foods, such as alfalfa, asparagus, avocados, cauliflower, grapefruit, and catfish.[22][23]
The effects of HMB on human skeletal muscle were first discovered by Steven L. Nissen at Iowa State University in the mid-1990s.[8][24] As of 2018, HMB has not been banned by the National Collegiate Athletic Association, World Anti-Doping Agency, or any other prominent national or international athletic organization.[25][26][27] In 2006, only about 2% of college student athletes in the United States used HMB as a dietary supplement.[19][28] As of 2017, HMB has found widespread use as an ergogenic supplement among young athletes.[29]
Uses
Available forms
HMB is sold as an over-the-counter dietary supplement in the free acid form, β-hydroxy β-methylbutyric acid (HMB-FA), and as a monohydrated calcium salt of the conjugate base, calcium β-hydroxy β-methylbutyrate monohydrate (HMB-Ca, CaHMB).[19][20] Since only a small fraction of HMB's metabolic precursor, L-leucine, is metabolized into HMB, pharmacologically active concentrations of the compound in blood plasma and muscle can only be achieved by supplementing HMB directly.[1][30][31] A healthy adult produces approximately 0.3 grams per day, while supplemental HMB is usually taken in doses of 3–6 grams per day.[17] HMB is sold at a cost of about US$30–50 per month when taken in doses of 3 grams per day.[16] HMB is also contained in several nutritional products and medical foods marketed by Abbott Laboratories (e.g., certain formulations of Ensure, Juven, and Myoplex),[8][21] and is present in insignificant quantities in certain foods, such as alfalfa, asparagus, avocados, cauliflower, grapefruit, and catfish.[22][23]
Medical
Supplemental HMB has been used in clinical trials as a treatment for preserving lean body mass in muscle wasting conditions, particularly sarcopenia, and has been studied in clinical trials as an adjunct therapy in conjunction with resistance exercise.[11][16][30] Based upon two medical reviews and a meta-analysis of seven randomized controlled trials, HMB supplementation can preserve or increase lean muscle mass and muscle strength in sarcopenic older adults.[note 2][11][12][13] HMB does not appear to significantly affect fat mass in older adults.[11][12] Preliminary clinical evidence suggests that HMB supplementation may also prevent muscle atrophy during bed rest.[11][29] A growing body of evidence supports the efficacy of HMB in nutritional support for reducing, or even reversing, the loss of muscle mass, muscle function, and muscle strength that occurs in hypercatabolic disease states such as cancer cachexia;[16][30][32] consequently, the authors of two 2016 reviews of the clinical evidence recommended that the prevention and treatment of sarcopenia and muscle wasting in general include supplementation with HMB, regular resistance exercise, and consumption of a high-protein diet.[16][30]
Clinical trials that used HMB for the treatment of muscle wasting have involved the administration of 3 grams of HMB per day under different dosing regimens.[16] According to one review, an optimal dosing regimen is to administer it in one 1 gram dose, three times a day, since this ensures elevated plasma concentrations of HMB throughout the day;[16] however, as of June 2016 the best dosing regimen for muscle wasting conditions is still being investigated.[30]
Some branded products that contain HMB (i.e., certain formulations of Ensure and Juven) are medical foods that are intended to be used to provide nutritional support under the care of a doctor in individuals with muscle wasting due to HIV/AIDS or cancer, to promote wound healing following surgery or injury, or when otherwise recommended by a medical professional.[sources 3] Juven, a nutrition product which contains 3 grams of HMB-Ca, 14 grams of L-arginine, and 14 grams of L-glutamine per two servings,[2] has been shown to improve lean body mass during clinical trials in individuals with AIDS and cancer, but not rheumatoid cachexia.[17][33][34] Further research involving the treatment of cancer cachexia with Juven over a period of several months is required to adequately determine treatment efficacy.[17][33]
Enhancing performance

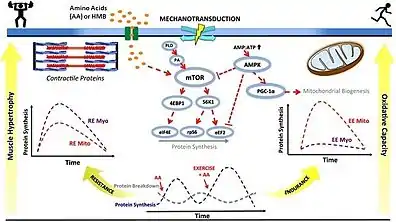
With an appropriate exercise program, dietary supplementation with 3 grams of HMB per day has been shown to increase exercise-induced gains in muscle size, muscle strength and power, and lean body mass, reduce exercise-induced skeletal muscle damage,[note 3] and expedite recovery from high-intensity exercise.[sources 2] Based upon limited clinical research, HMB supplementation may also improve aerobic exercise performance and increase gains in aerobic fitness when combined with high-intensity interval training.[12][14] These effects of HMB are more pronounced in untrained individuals and athletes who perform high intensity resistance or aerobic exercise.[1][12][14] In resistance-trained populations, the effects of HMB on muscle strength and lean body mass are limited.[37] HMB affects muscle size, strength, mass, power, and recovery in part by stimulating myofibrillar muscle protein synthesis and inhibiting muscle protein breakdown through various mechanisms, including the activation of mechanistic target of rapamycin complex 1 (mTORC1) and inhibition of proteasome-mediated proteolysis in skeletal muscles.[14][15]
The efficacy of HMB supplementation for reducing skeletal muscle damage from prolonged or high-intensity exercise is affected by the time that it is used relative to exercise.[1][36] The greatest reduction in skeletal muscle damage from a single bout of exercise has been shown to occur when HMB-Ca is ingested 1–2 hours prior to exercise or HMB-FA is ingested 30–60 minutes prior to exercise.[1]
In 2006, only about 2% of college student athletes in the United States used HMB as a dietary supplement.[19][28] As of 2017, HMB has found widespread use as an ergogenic supplement among athletes.[29] As of 2018, HMB has not been banned by the National Collegiate Athletic Association, World Anti-Doping Agency, or any other prominent national or international athletic organization.[25][26][27]
Side effects
The safety profile of HMB in adult humans is based upon evidence from clinical trials in humans and animal studies.[16][18] In humans, no adverse effects in young adults or older adults have been reported when HMB is taken in doses of 3 grams per day for up to a year.[16][17][18] Studies on young adults taking 6 grams of HMB per day for up to 2 months have also reported no adverse effects.[17][18] Studies with supplemental HMB on young, growing rats and livestock have reported no adverse effects based upon clinical chemistry or observable characteristics;[1][23] for humans younger than 18, there is limited data on the safety of supplemental HMB.[1] The human equivalent dose of HMB for the no-observed-adverse-effect level (NOAEL) that was identified in a rat model is approximately 0.4 g/kg of body weight per day.[note 4][18][23]
Two animal studies have examined the effects of HMB supplementation in pregnant pigs on the offspring and reported no adverse effects on the fetus.[23] No clinical testing with supplemental HMB has been conducted on pregnant women,[38] and pregnant and lactating women are advised not to take HMB by Metabolic Technologies, Inc., the company that grants licenses to include HMB in dietary supplements, due to a lack of safety studies.[38]
Pharmacology
• PA: phosphatidic acid
• mTOR: mechanistic target of rapamycin
• AMP: adenosine monophosphate
• ATP: adenosine triphosphate
• AMPK: AMP-activated protein kinase
• PGC‐1α: peroxisome proliferator-activated receptor gamma coactivator-1α
• S6K1: p70S6 kinase
• 4EBP1: eukaryotic translation initiation factor 4E-binding protein 1
• eIF4E: eukaryotic translation initiation factor 4E
• RPS6: ribosomal protein S6
• eEF2: eukaryotic elongation factor 2
• RE: resistance exercise; EE: endurance exercise
• Myo: myofibrillar; Mito: mitochondrial
• AA: amino acids
• HMB: β-hydroxy β-methylbutyric acid
• ↑ represents activation
• Τ represents inhibition
Pharmacodynamics
Several components of the signaling cascade that mediates the HMB-induced increase in human skeletal muscle protein synthesis have been identified in vivo.[14][15] Similar to HMB's metabolic precursor, L-leucine, HMB has been shown to increase protein synthesis in human skeletal muscle via phosphorylation of the mechanistic target of rapamycin (mTOR) and subsequent activation of mTORC1, which leads to protein biosynthesis in cellular ribosomes via phosphorylation of mTORC1's immediate targets (i.e., the p70S6 kinase and the translation repressor protein 4EBP1).[note 5][15][39] Supplementation with HMB in several non-human animal species has been shown to increase the serum concentration of growth hormone and insulin-like growth factor 1 (IGF-1) via an unknown mechanism, in turn promoting protein synthesis through increased mTOR phosphorylation.[1][16][23] Based upon limited clinical evidence in humans, supplemental HMB appears to increase the secretion of growth hormone and IGF-1 in response to resistance exercise.[14]
As of May 2016, the signaling cascade that mediates the HMB-induced reduction in muscle protein breakdown has not been identified in living humans, although it is well-established that it attenuates proteolysis in humans in vivo.[11][15] Unlike L-leucine, HMB attenuates muscle protein breakdown in an insulin-independent manner in humans.[note 6][15] HMB is believed to reduce muscle protein breakdown in humans by inhibiting the 19S and 20S subunits of the ubiquitin–proteasome system in skeletal muscle and by inhibiting apoptosis of skeletal muscle nuclei via unidentified mechanisms.[15][16]
Based upon animal studies, HMB appears to be metabolized within skeletal muscle into cholesterol, which may then be incorporated into the muscle cell membrane, thereby enhancing membrane integrity and function.[34][35] The effects of HMB on muscle protein metabolism may help stabilize muscle cell structure.[23] One review suggested that the observed HMB-induced reduction in the plasma concentration of muscle damage biomarkers (i.e., muscle enzymes such as creatine kinase and lactate dehydrogenase) in humans following intense exercise may be due to a cholesterol-mediated improvement in muscle cell membrane function.[note 3][23]
HMB has been shown to stimulate the proliferation, differentiation, and fusion of human myosatellite cells in vitro, which potentially increases the regenerative capacity of skeletal muscle, by increasing the protein expression of certain myogenic regulatory factors (e.g., myoD and myogenin) and gene transcription factors (e.g., MEF2).[1][17][42] HMB-induced human myosatellite cell proliferation in vitro is mediated through the phosphorylation of the mitogen-activated protein kinases ERK1 and ERK2.[17][23][42] HMB-induced human myosatellite differentiation and accelerated fusion of myosatellite cells into muscle tissue in vitro is mediated through the phosphorylation of Akt, a serine/threonine-specific protein kinase.[17][23][42]
Pharmacokinetics
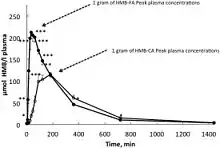
The free acid (HMB-FA) and monohydrated calcium salt (HMB-Ca) forms of HMB have different pharmacokinetics.[1][20] HMB-FA is more readily absorbed into the bloodstream and has a longer elimination half-life (3 hours) relative to HMB-Ca (2.5 hours).[1][20] Tissue uptake and utilization of HMB-FA is 25–40% higher than for HMB-Ca.[1][20] The fraction of an ingested dose that is excreted in urine does not differ between the two forms.[1]
After ingestion, HMB-Ca is converted to β-hydroxy β-methylbutyrate following dissociation of the calcium moiety in the gut.[1] When the HMB-Ca dosage form is ingested, the magnitude and time at which the peak plasma concentration of HMB occurs depends on the dose and concurrent food intake.[1] Higher HMB-Ca doses increase the rate of absorption, resulting in a peak plasma HMB level (Cmax) that is disproportionately greater than expected of a linear dose-response relationship and which occurs sooner relative to lower doses.[note 7][1] Consumption of HMB-Ca with sugary substances slows the rate of HMB absorption, resulting in a lower peak plasma HMB level that occurs later.[note 7][1]
HMB is eliminated via the kidneys, with roughly 10–40% of an ingested dose being excreted unchanged in urine.[1] The remaining 60–90% of the dose is retained in tissues or excreted as HMB metabolites.[1] The fraction of a given dose of HMB that is excreted unchanged in urine increases with the dose.[note 8][1]
Metabolism
Biosynthesis and metabolism of β-hydroxy β-methylbutyrate in humans
|
The metabolism of HMB is catalyzed by an uncharacterized enzyme which converts it to β-hydroxy β-methylbutyryl-CoA (HMB-CoA).[43][45] HMB-CoA is metabolized by either enoyl-CoA hydratase or another uncharacterized enzyme, producing β-methylcrotonyl-CoA (MC-CoA) or hydroxymethylglutaryl-CoA (HMG-CoA) respectively.[45] MC-CoA is then converted by the enzyme methylcrotonyl-CoA carboxylase to methylglutaconyl-CoA (MG-CoA), which is subsequently converted to HMG-CoA by methylglutaconyl-CoA hydratase.[45][46] HMG-CoA is then cleaved into acetyl-CoA and acetoacetate by HMG-CoA lyase or used in the production of cholesterol via the mevalonate pathway.[45]
Biosynthesis
HMB is synthesized in the human body through the metabolism of L-leucine, a branched-chain amino acid.[45] In healthy individuals, approximately 60% of dietary L-leucine is metabolized after several hours, with roughly 5% (2–10% range) of dietary L-leucine being converted to HMB.[16][45]
The vast majority of L-leucine metabolism is initially catalyzed by the branched-chain amino acid aminotransferase enzyme, producing α-ketoisocaproate (α-KIC).[45] α-KIC is mostly metabolized by the mitochondrial enzyme branched-chain α-ketoacid dehydrogenase, which converts it to isovaleryl-CoA.[45] Isovaleryl-CoA is subsequently metabolized by isovaleryl-CoA dehydrogenase and converted to MC-CoA, which is used in the synthesis of acetyl-CoA and other compounds.[45] During biotin deficiency, HMB can be synthesized from MC-CoA via enoyl-CoA hydratase and an unknown thioesterase enzyme,[43][44][47] which convert MC-CoA into HMB-CoA and HMB-CoA into HMB respectively.[44] A relatively small amount of α-KIC is metabolized in the liver by the cytosolic enzyme 4-hydroxyphenylpyruvate dioxygenase (KIC dioxygenase), which converts α-KIC to HMB.[45][48] In healthy individuals, this minor pathway – which involves the conversion of L-leucine to α-KIC and then HMB – is the predominant route of HMB synthesis.[45]
Chemistry
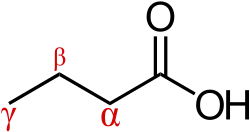

β-Hydroxy β-methylbutyric acid is a monocarboxylic β-hydroxy acid and natural product with the molecular formula C5H10O3.[49][50] At room temperature, pure β-hydroxy β-methylbutyric acid occurs as a transparent, colorless to light yellow liquid which is soluble in water.[6][51][52] β-Hydroxy β-methylbutyric acid is a weak acid with a pKa of 4.4.[5] Its refractive index () is 1.42.[5]
Chemical structure
β-Hydroxy β-methylbutyric acid is a member of the carboxylic acid family of organic compounds.[49] It is a structural analog of butyric acid with a hydroxyl functional group and a methyl substituent located on its beta carbon.[49][53] By extension, other structural analogs include β-hydroxybutyric acid and β-methylbutyric acid.[49][53]
Synthesis
A variety of synthetic routes to β-hydroxy β-methylbutyric acid have been developed. The first reported chemical syntheses approached HMB by oxidation of alkene, vicinal diol, and alcohol precursors:
- in 1877, Russian chemists Michael and Alexander Zaytsev reported the preparation of HMB by oxidation of 2-methylpent-4-en-2-ol with chromic acid (H2CrO4);[54]
- in 1880 and 1889, Schirokoff and Reformatsky (respectively) reported that the oxidative cleavage of the vicinal diol 4-methylpentane-1,2,4-triol with acidified potassium permanganate (KMnO4) yields HMB[55][56] – this result is closest related to the first synthesis as cold dilute KMnO4 oxidises alkenes to vicinal cis-diols which hot acid KMnO4 further oxidises to carbonyl-containing compounds, and the diol intermediate is not obtained when hot acidic conditions are used for alkene oxidation.[57] In other words, racemic 4-methylpentane-1,2,4-triol is a derivative of 2-methylpent-4-en-2-ol and β-hydroxy β-methylbutyric acid is a derivative of both; and,
- in 1892, Kondakow reported the preparation of HMB by permanganate oxidation of 3-methylbutane-1,3-diol.[58]

Depending on the experimental conditions, cycloaddition of acetone and ketene produces either β-isovalerolactone or 4,4-dimethyloxetan-2-one,[59][60] both of which hydrolyze under basic conditions to yield the conjugate base of HMB. The haloform reaction provides another pathway to HMB involving the exhaustive halogenation of the methyl-ketone region of diacetone alcohol with sodium hypobromite or sodium hypochlorite;[5][61][62] Diacetone alcohol is readily available from the aldol condensation of acetone.[61] An organometallic approach to HMB involves the carboxylation of tert-butyl alcohol with carbon monoxide and Fenton's reagent (hydrogen peroxide and ferrous iron).[5][63] Alternatively, HMB can be prepared through microbial oxidation of β-methylbutyric acid by the fungus Galactomyces reessii.[64]

Detection in body fluids
| Biofluid | Age group | Concentration | Sources | ||
|---|---|---|---|---|---|
| Mean | Range | Units | |||
| Blood plasma | Adults (18+) | 4.0 | 0–10.0 | μM | [49] |
| CSF | Adults (18+) | 4.0 | 2.0–6.0 | μM | [49] |
| Sarcoplasma | Adults (21–23) | 7.0 | 4.0–10.0 | μM | [15] |
| Breast milk | Adults (18+) | – | 42–164 | μg/L | [65] |
| Urine | Adults (18+) | – | 3.2–25.0 | μmol/mmol creatinine | [49] |
| Urine | Children (1–18) | – | 0–68 | μmol/mmol creatinine | [49] |
The concentration of naturally produced HMB has been measured in several human body fluids using nuclear magnetic resonance spectroscopy, liquid chromatography–mass spectrometry, and gas chromatography–mass spectrometry methods.[65][49] In the blood plasma and cerebrospinal fluid (CSF) of healthy adults, the average molar concentration of HMB has been measured at 4.0 micromolar (μM).[49] The average concentration of HMB in the intramuscular fluid of healthy men of ages 21–23 has been measured at 7.0 μM.[15] In the urine of healthy individuals of any age, the excreted urinary concentration of HMB has been measured in a range of 0–68 micromoles per millimole (μmol/mmol) of creatinine.[49] In the breast milk of healthy lactating women, HMB and L-leucine have been measured in ranges of 42–164 μg/L and 2.1–88.5 mg/L.[65] In comparison, HMB has been detected and measured in the milk of healthy cows at a concentration of <20–29 μg/L.[66] This concentration is far too low to be an adequate dietary source of HMB for obtaining pharmacologically active concentrations of the compound in blood plasma.[66]
In a study where participants consumed 2.42 grams of pure HMB-FA while fasting, the average plasma HMB concentration increased from a basal level of 5.1 μM to 408 μM after 30 minutes.[15] At 150 minutes post-ingestion, the average plasma HMB concentration among participants was 275 μM.[15]
Abnormal HMB concentrations in urine and blood plasma have been noted in several disease states where it may serve as a diagnostic biomarker, particularly in the case of metabolic disorders.[49] The following table lists some of these disorders along with the associated HMB concentrations detected in urine or blood plasma.[49]
| Medical condition | Biofluid | Age group | Concentration | Sources | ||
|---|---|---|---|---|---|---|
| Mean | Range | Units | ||||
| Biotinidase deficiency† | Blood | Adults (18+) | 9.5 | 0–19.0 | μM | [49] |
| Biotinidase deficiency† | Blood | Children (1–13) | 88.0 | 10.0–166.0 | μM | [49] |
| Biotinidase deficiency† | Urine | Children (1–13) | 275.0 | 50.0–500.0 | μmol/mmol creatinine | [49] |
| 3-Methylglutaconic aciduria (Type I)† | Urine | Children (1–13) | 200.0 | 150.0–250.0 | μmol/mmol creatinine | [49] |
| Eosinophilic esophagitis | Urine | Children (1–13) | 247.4 | 0–699.4 | μmol/mmol creatinine | [49] |
| Gastroesophageal reflux disease | Urine | Children (1–13) | 119.8 | 5.5–234.0 | μmol/mmol creatinine | [49] |
| HMG-CoA lyase deficiency† | Urine | Children (1–13) | 2030.0 | 60.0–4000.0 | μmol/mmol creatinine | [49] |
| MC-CoA carboxylase deficiency† | Urine | Children (1–13) | 30350.0 | 1700.0–59000.0 | μmol/mmol creatinine | [49] |
| A † indicates that the medical condition is a metabolic disorder. | ||||||
History
The first reported chemical synthesis of HMB was published in 1877 by the Russian chemists Michael and Alexander Zaytsev.[54] HMB was isolated from the bark of Erythrophleum couminga (a Madagascan tree) in 1941 by Leopold Ružička.[67] The earliest reported isolation of HMB as a human metabolite was by Tanaka and coworkers in 1968 from a patient with isovaleric acidemia.[68][69]
The effects of HMB on human skeletal muscle were first discovered by Steven L. Nissen at Iowa State University in the mid-1990s.[8][24] Nissen founded a company called Metabolic Technologies, Inc. (MTI) around the time of his discovery, which later acquired six HMB-related patents that the company has used to license the right to manufacture and incorporate HMB into dietary supplements.[24][70][71] When it first became available commercially in the late 1990s, HMB was marketed solely as an exercise supplement to help athletes and bodybuilders build muscle.[70] MTI subsequently developed two HMB-containing products, Juven and Revigor, to which Abbott Nutrition obtained the market rights in 2003 and 2008 respectively.[8][70] Since then, Abbott has marketed Juven as a medical food and the Revigor brand of HMB as an active ingredient in food products for athletes (e.g., certain formulations of Myoplex) and other medical foods (e.g., certain formulations of Ensure).[8][21][70]
See also
Notes
- Synonyms and alternate spellings include: beta-hydroxy beta-methylbutyric acid, 3-hydroxy-3-methylbutanoic acid (IUPAC name), 3-hydroxyisovaleric acid, and beta-hydroxyisovaleric acid.[7]
- The meta-analysis found that the average increase in muscle mass due to HMB supplementation in older adults was 0.35 kilograms (0.77 lb).[11] The 95% confidence interval for the estimated increase in muscle mass due to HMB supplementation is 0.11–0.59 kilograms (0.24–1.30 lb).[11]
The seven randomized controlled trials that were included in the meta-analysis contained a total of 147 older adults in the HMB treatment groups and 140 older adults in the control groups.[11] The seven trials had durations of 2–11 months and the average duration of the studies, weighted by their sample size, was approximately 6 months.[11] - The effect of HMB on skeletal muscle damage has been assessed in studies on humans using four different biomarkers of muscle damage or protein breakdown: serum creatine kinase, serum lactate dehydrogenase, urinary urea nitrogen, and urinary 3-methylhistidine.[1][19][35] When exercise intensity and volume are sufficient to cause skeletal muscle damage, such as during long-distance running or progressive overload, HMB supplementation has been demonstrated to attenuate the rise in these biomarkers by 20–60%.[1][19][36]
- The NOAEL was established based upon a 3-month study involving several groups of Sprague-Dawley rats that were administered different daily doses of HMB-FA.[18][23] No adverse effects were observed in any group that received HMB, so the highest daily dose of HMB that was administered in this study was identified as the NOAEL.[18][23]
- Approximately equal doses of pure HMB-FA (2.42 grams) and L-leucine (3.42 grams) do not produce statistically distinguishable anabolic effects, as measured by the fractional synthesis of myofibrillar proteins, in the skeletal muscle of living humans.[15][40] At 150 minutes post-ingestion, these doses of HMB-FA and L-leucine increased muscle protein synthesis by ~70% and ~110% respectively in one study.[15][40]
- At 150 minutes post-ingestion, a 2.42 gram dose of pure HMB-FA decreased skeletal muscle protein breakdown in living humans by 57% in one study.[15][40] The effect of L-leucine on muscle protein breakdown is entirely dependent upon insulin secretion and consequently was not measured in the same study.[15] By comparison, the insulin-dependent reduction in muscle protein breakdown following an entire meal that contains L-leucine and carbohydrates is ~50% on average.[15]
- In one study, ingestion of a 1 gram dose of HMB-Ca by healthy volunteers produced a peak plasma HMB level of 120 μM at 2 hours following ingestion, while ingestion of a 3 gram dose of HMB-Ca produced a peak plasma HMB level of 487 μM at 1 hour following ingestion.[1]
Consumption of 3 grams of HMB-Ca with 75 grams of glucose resulted in a lower peak plasma HMB level of 352 μM which occurred later at 2 hours following ingestion.[1] - In one study, ingestion of a 1 gram and 3 gram HMB dose resulted in the excretion of 14% and 28% of the dose as HMB in urine, respectively.[1]
- This reaction is catalyzed by an unknown thioesterase enzyme.[43][44]
References
- Wilson JM, Fitschen PJ, Campbell B, Wilson GJ, Zanchi N, Taylor L, Wilborn C, Kalman DS, Stout JR, Hoffman JR, Ziegenfuss TN, Lopez HL, Kreider RB, Smith-Ryan AE, Antonio J (February 2013). "International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB)". Journal of the International Society of Sports Nutrition. 10 (1): 6. doi:10.1186/1550-2783-10-6. PMC 3568064. PMID 23374455.
The [International Society of Sports Nutrition] has concluded the following. 1. HMB can be used to enhance recovery by attenuating exercise induced skeletal muscle damage in trained and untrained populations. ... 4. Thirty-eight mg·kg·BM−1 daily of HMB has been demonstrated to enhance skeletal muscle hypertrophy, strength, and power in untrained and trained populations when the appropriate exercise prescription is utilized. ... 8. HMB’s mechanisms of action include an inhibition and increase of proteolysis and protein synthesis, respectively. 9. Chronic consumption of HMB is safe in both young and old populations.
- "Product Information: Ensure Enlive Advanced Therapeutic Nutrition Shake" (PDF). Abbott Nutrition. 9 August 2016. Archived (PDF) from the original on 12 October 2016. Retrieved 22 August 2016.
• Use under medical supervision.
• HMB + protein for muscle health.
"Product Information: Juven" (PDF). Abbott Nutrition. 7 May 2016. Archived (PDF) from the original on 12 October 2016. Retrieved 22 August 2016.
• Administer orally or as a modular via feeding tube ...
• Use under medical supervision.
• Nutravigor® (CaHMB, calcium β-hydroxy-β-methylbutyrate) - "Safety data sheet: 3-Hydroxy-3-methyl butyric acid". Alfa Aesar. 23 March 2005. Archived from the original on 17 September 2016. Retrieved 9 November 2016.
- Coffman DD, Cramer R, Mochel WE (June 1958). "Syntheses by Free-radical Reactions. V. A New Synthesis of Carboxylic Acids". Journal of the American Chemical Society. 80 (11): 2882–2887. doi:10.1021/ja01544a072.
- "3-OH-isovaleric acid". ChemSpider. Royal Society of Chemistry. 2015. Archived from the original on 11 August 2016. Retrieved 10 August 2016.
Experimental Boiling Point: ... 128 °C / 7 mm ...
Experimental solubility:
Soluble in water - "beta-Hydroxyisovaleric acid". PubChem Compound. United States National Library of Medicine – National Center for Biotechnology Information. 3 February 2018. Archived from the original on 6 February 2018. Retrieved 6 February 2018.
Chemical Names: Beta-Hydroxyisovaleric acid; 3-Hydroxy-3-methylbutanoic acid; ... 3-Hydroxyisovaleric acid; 3-Hydroxy-3-methylbutyric acid
- Linn J (13 May 2013). "Proteins in Human Health and Performance". Iowa State University. Archived from the original on 27 August 2016. Retrieved 31 July 2016.
Dr. Nissen and his collaborator Dr. Naji N. Abumrad, Professor and Chair, Department of Surgery, Vanderbilt University, discovered beta-hydroxy-beta-methylbutyrate (HMB) and its beneficial effects on human health and performance. HMB is currently marketed nationally by Abbott Laboratories as Revigor™, which is a component of Ensure® Muscle Health, and Juven®, which is a nutritional beverage that is clinically shown to promote healing after injury or surgery.
- Khamsi R (May 2013). "Rethinking the formula". Nature Medicine. 19 (5): 525–529. doi:10.1038/nm0513-525. PMID 23652097. S2CID 205379191.
The questions about what defines a medical food will likely grow as the market does—and that market now extends far beyond PKU and other inherited metabolic disorders. ... Abbott Nutrition's Juven provides nutrients to people with HIV or AIDS experiencing excessive weight loss due to disease
- "JUVEN Added to Abbott Laboratories' Nutritional Product Line for People With Cancer, HIV/AIDS and Wounds/Pressure Ulcers". PR Newswire. Abbott Laboratories. 12 March 2004. Archived from the original on 20 December 2016. Retrieved 11 December 2016.
- Wu H, Xia Y, Jiang J, Du H, Guo X, Liu X, Li C, Huang G, Niu K (September 2015). "Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: a systematic review and meta-analysis". Archives of Gerontology and Geriatrics. 61 (2): 168–175. doi:10.1016/j.archger.2015.06.020. PMID 26169182.
Overall, this meta-analysis indicates that HMB can prevent lean body mass loss in older adults. But the effects of HMB on muscle strength and physical function appears to vary in different populations. Additional well-designed clinical studies are necessary to confirm the effectiveness of HMB in the prevention of loss of muscle strength and physical function. ... Mechanisms underlying the role of HMB in muscle regeneration have also been explored: results indicated that HMB enhances protein synthesis via upregulation of anabolic signaling pathways and attenuate proteolysis via downregulation of catabolic signaling pathways (Wilkinson et al., 2013).
- Holeček M (August 2017). "Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions". Journal of Cachexia, Sarcopenia and Muscle. 8 (4): 529–541. doi:10.1002/jcsm.12208. PMC 5566641. PMID 28493406.
The reports summarized here indicate that HMB provides a number of benefits to subjects involved in strength-power and endurance sports. The effects on muscle mass and strength, particularly during resistance training, are likely related to the suppression of proteolysis and a positive effect on protein synthesis. Its benefits in aerobic performance are probably more associated with improved mitochondrial biogenesis and fat oxidation. Favourable effects on the recovery from exercise-induced damage may be related to the role of HMB as a precursor of cholesterol, which modulates membrane fluidity and affects ion channels, and membrane excitability. ... Studies have demonstrated that HMB can prevent the development of sarcopenia in elderly subjects and that the optimal action of HMB on muscle growth and strength occurs when it is combined with exercise.
- Rossi AP, D'Introno A, Rubele S, Caliari C, Gattazzo S, Zoico E, Mazzali G, Fantin F, Zamboni M (October 2017). "The Potential of β-Hydroxy-β-Methylbutyrate as a New Strategy for the Management of Sarcopenia and Sarcopenic Obesity". Drugs & Aging. 34 (11): 833–840. doi:10.1007/s40266-017-0496-0. PMID 29086232. S2CID 4284897.
Clinical trials performed in older adults confirm that HMB can attenuate the progression of sarcopenia in elderly subjects. HMB supplementation results in an increase in skeletal muscle mass and strength in the elderly and its effect is even greater when combined with physical exercise.
- Silva VR, Belozo FL, Micheletti TO, Conrado M, Stout JR, Pimentel GD, Gonzalez AM (September 2017). "β-hydroxy-β-methylbutyrate free acid supplementation may improve recovery and muscle adaptations after resistance training: a systematic review" (PDF). Nutrition Research. 45: 1–9. doi:10.1016/j.nutres.2017.07.008. hdl:11449/170023. PMID 29037326.
HMB's mechanisms of action are generally considered to relate to its effect on both muscle protein synthesis and muscle protein breakdown (Figure 1) [2, 3]. HMB appears to stimulate muscle protein synthesis through an up-regulation of the mammalian/mechanistic target of rapamycin complex 1 (mTORC1), a signaling cascade involved in coordination of translation initiation of muscle protein synthesis [2, 4]. Additionally, HMB may have antagonistic effects on the ubiquitin–proteasome pathway, a system that degrades intracellular proteins [5, 6]. Evidence also suggests that HMB promotes myogenic proliferation, differentiation, and cell fusion [7]. ... Exogenous HMB-FA administration has shown to increase intramuscular anabolic signaling, stimulate muscle protein synthesis, and attenuate muscle protein breakdown in humans [2].
- Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM, Etheridge T, Rathmacher JA, Smith K, Szewczyk NJ, Atherton PJ (June 2013). "Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism". The Journal of Physiology. 591 (11): 2911–2923. doi:10.1113/jphysiol.2013.253203. PMC 3690694. PMID 23551944.
The stimulation of MPS through mTORc1-signalling following HMB exposure is in agreement with pre-clinical studies (Eley et al. 2008). ... Furthermore, there was clear divergence in the amplitude of phosphorylation for 4EBP1 (at Thr37/46 and Ser65/Thr70) and p70S6K (Thr389) in response to both Leu and HMB, with the latter showing more pronounced and sustained phosphorylation. ... Nonetheless, as the overall MPS response was similar, this cellular signalling distinction did not translate into statistically distinguishable anabolic effects in our primary outcome measure of MPS. ... Interestingly, although orally supplied HMB produced no increase in plasma insulin, it caused a depression in MPB (−57%). Normally, postprandial decreases in MPB (of ~50%) are attributed to the nitrogen-sparing effects of insulin since clamping insulin at post-absorptive concentrations (5 μU ml−1) while continuously infusing AAs (18 g h−1) did not suppress MPB (Greenhaff et al. 2008), which is why we chose not to measure MPB in the Leu group, due to an anticipated hyperinsulinaemia (Fig. 3C). Thus, HMB reduces MPB in a fashion similar to, but independent of, insulin. These findings are in-line with reports of the anti-catabolic effects of HMB suppressing MPB in pre-clinical models, via attenuating proteasomal-mediated proteolysis in response to LPS (Eley et al. 2008).
- Brioche T, Pagano AF, Py G, Chopard A (August 2016). "Muscle wasting and aging: Experimental models, fatty infiltrations, and prevention" (PDF). Molecular Aspects of Medicine. 50: 56–87. doi:10.1016/j.mam.2016.04.006. PMID 27106402.
In conclusion, HMB treatment clearly appears to be a safe potent strategy against sarcopenia, and more generally against muscle wasting, because HMB improves muscle mass, muscle strength, and physical performance. It seems that HMB is able to act on three of the four major mechanisms involved in muscle deconditioning (protein turnover, apoptosis, and the regenerative process), whereas it is hypothesized to strongly affect the fourth (mitochondrial dynamics and functions). Moreover, HMB is inexpensive (~30– 50 US dollars per month at 3 g per day) and may prevent osteopenia (Bruckbauer and Zemel, 2013; Tatara, 2009; Tatara et al., 2007, 2008, 2012) and decrease cardiovascular risks (Nissen et al., 2000). For all these reasons, HMB should be routinely used in muscle-wasting conditions especially in aged people. ... 3 g of CaHMB taken three times a day (1 g each time) is the optimal posology, which allows for continual bioavailability of HMB in the body (Wilson et al., 2013)
- Molfino A, Gioia G, Rossi Fanelli F, Muscaritoli M (December 2013). "Beta-hydroxy-beta-methylbutyrate supplementation in health and disease: a systematic review of randomized trials". Amino Acids. 45 (6): 1273–1292. doi:10.1007/s00726-013-1592-z. PMID 24057808. S2CID 8688823.
Normally, an individual metabolizes 60 g of L-LEU to obtain 3 g of HMB but a 70 kg person produces 0.2–0.4 g of HMB per day, depending on the dose of LEU in the diet (Van Koevering and Nissen 1992). ... The usual dose of 3 g/day may be routinely recommended to maintain or improve muscle mass and function in health and disease. The safety profile of HMB is unequivocal. ... These results show that HMB/ARG/GLN can be safely used to treat AIDS- and cancer-related muscle wasting
- Borack MS, Volpi E (December 2016). "Efficacy and Safety of Leucine Supplementation in the Elderly". The Journal of Nutrition. 146 (12): 2625S–2629S. doi:10.3945/jn.116.230771. PMC 5118760. PMID 27934654.
One study tested the safety of HMB for long-term use in rats. Fuller et al. (50) conducted a 91-d study with the use of Sprague-Dawley rats that tested the safety of β-hydroxy-β-methylbutyric free acid (HMBFA). This new form of HMB results in higher HMB serum concentrations than CaHMB. In this study, rats were administered an HMBFA intervention of 0%, 0.8%, 1.6%, or 4% of the diet by body weight. The highest dose is the equivalent of ~400 mg ⋅ kg−1 ⋅ d−1 for humans. No adverse advents were observed for any treatment group. Similarly, blood and urine analyses were within the normal range in all groups, with no group differences. The authors concluded that HMBFA was safe for consumption in a rat model. ... No serious side effects have been reported with leucine, EAA, or HMB supplementation; and the health risks associated with these supplements are few and predictable.
- Momaya A, Fawal M, Estes R (April 2015). "Performance-enhancing substances in sports: a review of the literature". Sports Medicine. 45 (4): 517–531. doi:10.1007/s40279-015-0308-9. PMID 25663250. S2CID 45124293.
Currently, HMB is available as an over-the-counter supplement. The drug is not tested for nor banned by any sporting organization. ... Wilson et al. [91] demonstrated that when non-resistance trained males received HMB pre-exercise, the rise of lactate dehydrogenase (LDH) levels reduced, and HMB tended to decrease soreness. Knitter et al. [92] showed a decrease in LDH and creatine phosphokinase (CPK), a byproduct of muscle breakdown, by HMB after a prolonged run. ... The utility of HMB does seem to be affected by timing of intake prior to workouts and dosage [97]. Further, chronic consumption of HMB appears safe [97]. ... No serious adverse effects from HMB consumption have been reported.
- Fuller JC, Sharp RL, Angus HF, Khoo PY, Rathmacher JA (November 2015). "Comparison of availability and plasma clearance rates of β-hydroxy-β-methylbutyrate delivery in the free acid and calcium salt forms". primary source. The British Journal of Nutrition. 114 (9): 1403–1409. doi:10.1017/S0007114515003050. PMID 26373270.
Recently, the free acid form of HMB (HMB-FA) has become commercially available in capsule form (gelcap). The current study was conducted to compare the bioavailability of HMB using the two commercially available capsule forms of HMB-FA and Ca-HMB. ... In conclusion, HMB-FA in capsule form improves clearance rate and availability of HMB compared with Ca-HMB in capsule form.
- "Abbott Nutrition Overview" (PDF). Abbott. Abbott Laboratories. Archived from the original (PDF) on 3 September 2016. Retrieved 3 September 2016.
- Wilson GJ, Wilson JM, Manninen AH (January 2008). "Effects of beta-hydroxy-beta-methylbutyrate (HMB) on exercise performance and body composition across varying levels of age, sex, and training experience: A review". Nutrition & Metabolism. 5: 1. doi:10.1186/1743-7075-5-1. PMC 2245953. PMID 18173841.
- Szcześniak KA, Ostaszewski P, Fuller JC, Ciecierska A, Sadkowski T (June 2015). "Dietary supplementation of β-hydroxy-β-methylbutyrate in animals – a review". Journal of Animal Physiology and Animal Nutrition. 99 (3): 405–417. doi:10.1111/jpn.12234. PMID 25099672.
Cholesterol is a major component of the cell membrane, and sarcolemma is the one that relies mainly on de novo synthesis of cholesterol. This is important under stressful conditions when muscle cells may lack the capacity to produce adequate amounts of the cholesterol that is essential to proper functioning of cell membranes. Many biochemical studies have shown that HMB may be a precursor of cholesterol synthesis (Bachhawat et al., 1955; Bloch et al., 1954; Coon et al., 1955; Adamson and Greenberg, 1955; Gey et al., 1957). According to pertinent literature, HMB carbon is incorporated into cholesterol. Therefore, increased intramuscular HMB concentrations may provide readily available substrate for the cholesterol synthesis that is needed to form and stabilize the sarcolemma. ... It is known that HMB supplementation decreases post-exercise levels of enzymes, indicating muscle damage, such as creatinine phosphokinase (CK) and lactate dehydrogenase (LDH), which suggests an enhancement of the muscle cell membrane function. This was shown in numerous studies in humans undergoing both resistance and endurance training (Wilson et al., 2013) ... In theory, HMB use as a precursor to cholesterol could aid in stabilizing muscle cell membranes; however, this has not been confirmed by research studies. The effect of HMB on protein metabolism may in fact help stabilize muscle structure more than any effect HMB may have on cholesterol metabolism in the cell.
- Fitzgerald M (May 2014). Diet Cults: The Surprising Fallacy at the Core of Nutrition Fads and a Guide to Healthy Eating for the Rest of Us. Pegasus Books. p. 148. ISBN 978-1-60598-595-4. Retrieved 31 July 2016.
HMB was discovered in the mid-1990s by Steve Nissen, a researcher at Iowa State University
- Rippe JM (March 2013). "Beta-Hydroxy beta-methylbutyrate". Lifestyle Medicine (2nd ed.). CRC Press. p. 724. ISBN 978-1-4398-4544-8. Archived from the original on 22 March 2018. Retrieved 15 August 2016.
- "Prohibited List (January 2018)" (PDF). World Anti-Doping Agency. Archived (PDF) from the original on 22 October 2017. Retrieved 17 December 2017.
- "2018–19 NCAA Banned Drugs List". National Collegiate Athletic Association. 10 June 2015. Retrieved 22 August 2018.
- The NCAA Research Staff (January 2006). "NCAA Study of Substance Use Habits of College Student-Athletes" (PDF). National Collegiate Athletic Association. p. 7. Archived (PDF) from the original on 10 May 2016. Retrieved 24 June 2016.
- Cruz-Jentoft AJ (2018). "Beta-hydroxy-beta-methyl butyrate (HMB): From experimental data to clinical evidence in sarcopenia". Current Protein & Peptide Science. 18 (7): 668–672. doi:10.2174/1389203718666170529105026. PMID 28554316.
HMB is widely used as an ergogenic supplement by young athletes. ... This study shows that in healthy older adult, HMB supplementation may preserve muscle mass during 10 days of bed rest. These results are encouraging, but need to be confirmed by other groups.
- Argilés JM, Campos N, Lopez-Pedrosa JM, Rueda R, Rodriguez-Mañas L (September 2016). "Skeletal Muscle Regulates Metabolism via Interorgan Crosstalk: Roles in Health and Disease". Journal of the American Medical Directors Association. 17 (9): 789–796. doi:10.1016/j.jamda.2016.04.019. PMID 27324808.
Studies suggest dietary protein and leucine or its metabolite β-hydroxy β-methylbutyrate (HMB) can improve muscle function, in turn improving functional performance. ... These have identified the leucine metabolite β-hydroxy β-methylbutyrate (HMB) as a potent stimulator of protein synthesis as well as an inhibitor of protein breakdown in the extreme case of cachexia. ... A growing body of evidence suggests HMB may help slow, or even reverse, the muscle loss experienced in sarcopenia and improve measures of muscle strength. ... However, dietary leucine does not provide a large amount of HMB: only a small portion, as little as 5%, of catabolized leucine is metabolized into HMB. ... Thus, although dietary leucine itself can lead to a modest stimulation of protein synthesis by producing a small amount of HMB, direct ingestion of HMB more potently affects such signaling, resulting in demonstrable muscle mass accretion. ... Indeed, a vast number of studies have found that supplementation of HMB to the diet may reverse some of the muscle loss seen in sarcopenia and in hypercatabolic disease. ... The overall treatment of muscle atrophy should include dietary supplementation with HMB, although the optimal dosage for each condition is still under investigation. ...
Figure 4: Treatments for sarcopenia. It is currently recommended that patients at risk of or suffering from sarcopenia consume a diet high in protein, engage in resistance exercise, and take supplements of the leucine metabolite HMB. - Landi F, Calvani R, Tosato M, Martone AM, Ortolani E, Savera G, D'Angelo E, Sisto A, Marzetti E (May 2016). "Protein Intake and Muscle Health in Old Age: From Biological Plausibility to Clinical Evidence". Nutrients. 8 (5): 295. doi:10.3390/nu8050295. PMC 4882708. PMID 27187465.
HMB is an active leucine metabolite which activates the mTOR signaling pathway in muscle. Following its absorption, dietary leucine is converted into α-ketoisocaproate (KIC), which is further metabolized into either isovaleryl-CoA or HMB. Under normal conditions, the majority of KIC is converted into isovaleryl-CoA, while only approximately 5% of leucine is metabolized to HMB. This implies that, in order to reach pharmacological levels of HMB, this compound needs to be administered directly, rather than via increasing leucine dosage. ... HMB exerts its effects through protective, anticatabolic mechanisms and directly influences protein synthesis. HMB has also been shown to stabilize the muscle cell membrane, to modulate protein degradation and to up-regulate protein synthesis [68].
- Mullin GE (February 2014). "Nutrition supplements for athletes: potential application to malnutrition". Nutrition in Clinical Practice. 29 (1): 146–147. doi:10.1177/0884533613516130. PMID 24336486.
There are a number of nutrition products on the market that are touted to improve sports performance. HMB appears to be the most promising and to have clinical applications to improve muscle mass and function. Continued research using this nutraceutical to prevent and/or improve malnutrition in the setting of muscle wasting is warranted.
- Mochamat, Cuhls H, Marinova M, Kaasa S, Stieber C, Conrad R, Radbruch L, Mücke M (July 2016). "A systematic review on the role of vitamins, minerals, proteins, and other supplements for the treatment of cachexia in cancer: a European Palliative Care Research Centre cachexia project". Journal of Cachexia, Sarcopenia and Muscle. 8 (1): 25–39. doi:10.1002/jcsm.12127. PMC 5326814. PMID 27897391.
Looking at studies with proteins and other dietary supplements the combination of HMB, arginine, and glutamine showed interesting results ... In one study, 32 patients gained an average of about 2 kg of body weight.[21] This study was one of three studies confirming the positive effects of this combination in a variety of diagnoses/conditions such as HIV/AIDS patients and healthy adults.[40] Another study, on a far larger sample base of around 470 cancer patients, found no significant difference with regard to LBM after 8 weeks however a strong trend in the direction of an increase in LBM as measured by both bio-impedance and skin-fold measurements.[22] In summary, the effect of the combination of HMB, arginine, and glutamine on weight gain should be investigated in further studies on cancer patients investigating time periods of several months.
- Rahman A, Wilund K, Fitschen PJ, Jeejeebhoy K, Agarwala R, Drover JW, Mourtzakis M (July 2014). "Elderly persons with ICU-acquired weakness: the potential role for β-hydroxy-β-methylbutyrate (HMB) supplementation?". JPEN. Journal of Parenteral and Enteral Nutrition. 38 (5): 567–575. doi:10.1177/0148607113502545. PMID 24072740.
More than 20 publications in humans have demonstrated benefit with HMB supplementation associated with increased lean body mass without fat gain, improved markers of muscle strength, and decreased onset of muscle soreness with training and reduced markers of muscle damage. ... One proposed cellular mechanism for HMB is principally through stabilization of the cholesterol membrane in muscle cells. HMB is metabolized to β-hydroxy-β-methylglutaryl-coenzyme A (HMG-CoA) in the cytosol of muscle cells, which in turn is converted to cholesterol. ... Muscle produces its own cholesterol to maintain the integrity of the cell membrane, typically from HMG-CoA, because it cannot supply its cholesterol needs via absorption from the circulation.
- Luckose F, Pandey MC, Radhakrishna K (2015). "Effects of amino acid derivatives on physical, mental, and physiological activities". Critical Reviews in Food Science and Nutrition. 55 (13): 1793–1807. doi:10.1080/10408398.2012.708368. PMID 24279396. S2CID 22657268.
HMB, a derivative of leucine, prevents muscle damage and increases muscle strength by reducing exercise-induced proteolysis in muscles and also helps in increasing lean body mass. ... HMB is converted to HMB-CoA which is then used for the synthesis of cholesterol in muscle cells (Nissen and Abumrad, 1997). Cholesterol is needed for the growth, repair, and stabilization of cellular membranes during exercise (Chen, 1984). ... The meta analysis studies and the individual studies conducted support the use of HMB as an effective aid to increase body strength, body composition, and to prevent muscle damage during resistance training.
- Rahimi MH, Mohammadi H, Eshaghi H, Askari G, Miraghajani M (2018). "The Effects of Beta-Hydroxy-Beta-Methylbutyrate Supplementation on Recovery Following Exercise-Induced Muscle Damage: A Systematic Review and Meta-Analysis". Journal of the American College of Nutrition. 37 (7): 640–649. doi:10.1080/07315724.2018.1451789. PMID 29676656. S2CID 4991601.
The current evidence revealed a time-dependent effect of HMB in reducing LDH and CK serum levels among adults. HMB, therefore, may be seen as a priority muscle damage recovery agent in interventions.
- Sanchez-Martinez J, Santos-Lozano A, Garcia-Hermoso A, Sadarangani KP, Cristi-Montero C (July 2018). "Effects of beta-hydroxy-beta-methylbutyrate supplementation on strength and body composition in trained and competitive athletes: A meta-analysis of randomized controlled trials". Journal of Science and Medicine in Sport. 21 (7): 727–735. doi:10.1016/j.jsams.2017.11.003. PMID 29249685.
- "Who should not take HMB?". Metabolic Technologies, Inc. 11 September 2014. Archived from the original on 26 August 2016. Retrieved 23 August 2016.
Pregnant or lactating women are advised against taking HMB because safety studies have not yet been conducted for these populations.
- Brook MS, Wilkinson DJ, Phillips BE, Perez-Schindler J, Philp A, Smith K, Atherton PJ (January 2016). "Skeletal muscle homeostasis and plasticity in youth and ageing: impact of nutrition and exercise". Acta Physiologica. 216 (1): 15–41. doi:10.1111/apha.12532. PMC 4843955. PMID 26010896.
The mechanisms underlying the anabolic effects of food intake involve both the stimulation of MPS (Rennie et al. 1982) and suppression of MPB (Wilkes et al. 2009). The potent increase in MPS is driven almost entirely by essential amino acids (EAAs) (Smith et al. 1992), with the branched chain AA (BCAA: leucine, isoleucine and valine), in particular leucine [and its metabolite(s), e.g. β‐hydroxy β‐methylbutyric acid (HMB) (Van Koevering & Nissen 1992)] being central to these effects (Wilkinson et al. 2013). Although the mechanisms underlying the unique anabolic properties of leucine are incompletely defined, recent work in yeast and cultured mammalians cells has demonstrated that leucyl tRNA synthetase is upstream of activating the hitherto ‘cellular AA sensor’, the mechanistic target of rapamycin complex 1 (mTORC1) in response to leucine (Bonfils et al. 2012, Han et al. 2012). This was reaffirmed by experiments showing that of all the EAAs, leucine is the most effective EAA in increasing the activity (i.e. phosphorylation) of mTORC1 (Atherton et al. 2010b) and its substrates.
- Phillips SM (July 2015). "Nutritional supplements in support of resistance exercise to counter age-related sarcopenia". Advances in Nutrition. 6 (4): 452–460. doi:10.3945/an.115.008367. PMC 4496741. PMID 26178029.
- Kornasio R, Riederer I, Butler-Browne G, Mouly V, Uni Z, Halevy O (May 2009). "Beta-hydroxy-beta-methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3K/Akt pathways". primary source. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1793 (5): 755–763. doi:10.1016/j.bbamcr.2008.12.017. PMID 19211028.
- "KEGG Reaction: R10759". Kyoto Encyclopedia of Genes and Genomes. Kanehisa Laboratories. Archived from the original on 1 July 2016. Retrieved 24 June 2016.
- Mock DM, Stratton SL, Horvath TD, Bogusiewicz A, Matthews NI, Henrich CL, Dawson AM, Spencer HJ, Owen SN, Boysen G, Moran JH (November 2011). "Urinary excretion of 3-hydroxyisovaleric acid and 3-hydroxyisovaleryl carnitine increases in response to a leucine challenge in marginally biotin-deficient humans". primary source. The Journal of Nutrition. 141 (11): 1925–1930. doi:10.3945/jn.111.146126. PMC 3192457. PMID 21918059.
Reduced activity of MCC impairs catalysis of an essential step in the mitochondrial catabolism of the BCAA leucine. Metabolic impairment diverts methylcrotonyl CoA to 3-hydroxyisovaleryl CoA in a reaction catalyzed by enoyl-CoA hydratase (22, 23). 3-Hydroxyisovaleryl CoA accumulation can inhibit cellular respiration either directly or via effects on the ratios of acyl CoA:free CoA if further metabolism and detoxification of 3-hydroxyisovaleryl CoA does not occur (22). The transfer to carnitine by 4 carnitine acyl-CoA transferases distributed in subcellular compartments likely serves as an important reservoir for acyl moieties (39–41). 3-Hydroxyisovaleryl CoA is likely detoxified by carnitine acetyltransferase producing 3HIA-carnitine, which is transported across the inner mitochondrial membrane (and hence effectively out of the mitochondria) via carnitine-acylcarnitine translocase (39). 3HIA-carnitine is thought to be either directly deacylated by a hydrolase to 3HIA or to undergo a second CoA exchange to again form 3-hydroxyisovaleryl CoA followed by release of 3HIA and free CoA by a thioesterase.
- Kohlmeier M (May 2015). "Leucine". Nutrient Metabolism: Structures, Functions, and Genes (2nd ed.). Academic Press. pp. 385–388. ISBN 978-0-12-387784-0. Archived from the original on 22 March 2018. Retrieved 6 June 2016.
Energy fuel: Eventually, most Leu is broken down, providing about 6.0kcal/g. About 60% of ingested Leu is oxidized within a few hours ... Ketogenesis: A significant proportion (40% of an ingested dose) is converted into acetyl-CoA and thereby contributes to the synthesis of ketones, steroids, fatty acids, and other compounds
Figure 8.57: Metabolism of L-leucine Archived 22 March 2018 at the Wayback Machine - "Leucine metabolism". BRENDA. Technische Universität Braunschweig. Archived from the original on 17 August 2016. Retrieved 12 August 2016.
- "KEGG Reaction: R04137". Kyoto Encyclopedia of Genes and Genomes. Kanehisa Laboratories. Archived from the original on 1 July 2016. Retrieved 24 June 2016.
- "Homo sapiens: 4-hydroxyphenylpyruvate dioxygenase reaction". MetaCyc. SRI International. 20 August 2012. Retrieved 6 June 2016.
- "3-Hydroxyisovaleric acid". HMDB Version 4.0. Human Metabolome Database. 7 December 2017. Archived from the original on 5 December 2017. Retrieved 26 December 2017.
- "3-hydroxyisovalerate". Chemical Entities of Biological Interest. European Bioinformatics Institute. 16 September 2014. Archived from the original on 1 December 2017. Retrieved 20 August 2016.
- WO application 2015094925, White TO, "Stable liquid filled hard capsule comprising beta-hydroxy-beta-methylbutyric acid", published 25 June 2015, assigned to Capsugel Belgium Nv
- "Beta-Hydroxyisovaleric acid". ChemicalBook. Archived from the original on 21 August 2016. Retrieved 20 August 2016.
- "3-hydroxyisovaleric acid". Chemical Entities of Biological Interest. European Bioinformatics Institute. 23 October 2015. Archived from the original on 12 March 2016. Retrieved 20 August 2016.
- The earliest citation for the synthesis of β-hydroxy β-methylbutyric acid in the Reaxys chemical database as of September 2016 is:
Saytzeff M, Saytzeff A (1877). "Synthese des Allyldimethylcarbinols" [Synthesis of allyldimethylcarbinols]. Justus Liebig's Annalen der Chemie (in German). 185 (2–3): 151–169. doi:10.1002/jlac.18771850204. - Schirokoff A (January 1881). "Ueber die β-Dipropyl- und β-Diäthyläthylenmilchsäure und über die Oxydation des Allyldimethylcarbinols und Diallylcarbinols mit übermangansaurem Kalium" [On the β-dipropyl- and β-diethylenyl-lactic acid, and on the oxidation of the allyl dimethylcarbinol and diallylcarbinol with excess potassium]. Journal für Praktische Chemie (in German). 23 (1): 196–208. doi:10.1002/prac.18810230115.
- Reformatzky B (30 October 1889). "Synthese einiger Glycerine mittelst unterchloriger Säure" [Synthesis of some glycerol by hypochlorous acid]. Journal für Praktische Chemie (in German). 40 (1): 396–419. doi:10.1002/prac.18890400137.
- McMurry, John E. (2010). "Oxidation of Alkenes: Epoxidation, Hydroxylation, and Cleavage". Fundamentals of Organic Chemistry (7th ed.). Cengage Learning. pp. 124–126, 142. ISBN 9781439049716.
- Kondakow J (1892). "On the action of mineral acids on dimethylallyls". Zhurnal Russkago Fiziko-Khimicheskago Obshchestva (Journal of the Russian Physico-Chemical Society) (in Russian). 1: 508–513. abstracted by Grosset (1893). "Ueber die Einwirkung von Mineralsauren auk Dimethylallen" [On the action of mineral acids on dimethylallyls]. Berichte der Deutschen Chemischen Gesellschaft (in German). 26 (4): 96. doi:10.1002/cber.18930260412.
- Gresham TL, Jansen JE, Shaver FW, Beears WL (January 1954). "β-Propiolactone. XIV. β-Isovalerolactone". Journal of the American Chemical Society. 76 (2): 486–488. doi:10.1021/ja01631a045.
- WO application 2012140276, Noti C, Schmid L, Rittiner B, Hanselmann P, Bierstedt A, "Process for the preparation of 3-hydroxy-3-methylbutyric acid or its calcium salts", published 10 January 2013, assigned to Lonza Ltd
- Kohn M (September 1903). "Zur Kenntnis des Diacetonalkohols und des Mesityloxyds" [Knowledge of diacetone alkohols and mesityl oxide]. Monatshefte für Chemie und Verwandte Teile Anderer Wissenschaften. 24 (9): 765–772. doi:10.1007/BF01526057. S2CID 96317019.
- Doraiswamy LK (February 2001). "Example 5.2". Organic Synthesis Engineering. New York: Oxford University Press. pp. 102–124. ISBN 978-0-19-509689-7.
- Kochi JK (December 2012). "Homolytic Mechanism in Metal Catalysis". Organometallic Mechanisms and Catalysis: The Role of Reactive Intermediates in Organic Processes. New York: Elsevier. p. 67. ISBN 978-0-323-14410-0. Archived from the original on 22 March 2018.
- Lee IY, Nissen SL, Rosazza JP (November 1997). "Conversion of beta-methylbutyric acid to beta-hydroxy-beta-methylbutyric acid by Galactomyces reessii". primary source. Applied and Environmental Microbiology. 63 (11): 4191–4195. doi:10.1128/AEM.63.11.4191-4195.1997. PMC 168736. PMID 9361403.
- Ehling S, Reddy TM (September 2015). "Direct Analysis of Leucine and Its Metabolites β-Hydroxy-β-methylbutyric Acid, α-Ketoisocaproic Acid, and α-Hydroxyisocaproic Acid in Human Breast Milk by Liquid Chromatography-Mass Spectrometry". primary source. Journal of Agricultural and Food Chemistry. 63 (34): 7567–7573. doi:10.1021/acs.jafc.5b02563. PMID 26271627.
- Ehling S, Reddy TM (February 2014). "Investigation of the presence of β-hydroxy-β-methylbutyric acid and α-hydroxyisocaproic acid in bovine whole milk and fermented dairy products by a validated liquid chromatography-mass spectrometry method". primary source. Journal of Agricultural and Food Chemistry. 62 (7): 1506–1511. doi:10.1021/jf500026s. PMID 24495238.
- Ružička L, Dalma G, Engel BG, Scott WE (1941). "Zur Kenntnis der Erythrophleum-Alkaloide. (5. Mitteilung). Identifizierung der niedermolekularen Spaltsäure des Coumingins" [Concerning erythrophleum alkaloids. (5th Communication). Identification of the low molecular weight cleavage acids from coumingin]. Helvetica Chimica Acta (in German). 24 (1): 1449–1458. doi:10.1002/hlca.194102401171.
- Tanaka K, Orr JC, Isselbacher KJ (May 1968). "Identification of beta-hydroxyisovaleric acid in the urine of a patient with isovaleric acidemia". primary source. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 152 (3): 638–41. doi:10.1016/0005-2760(68)90107-0. PMID 5656832.
- Tanaka K (1975). "Disorders of organic acid metabolism". In Gaull GE (ed.). Biology of Brain Dysfunction Volume 3. Boston, MA: Springer US. pp. 145–214. doi:10.1007/978-1-4684-2673-1_3. ISBN 978-1-4684-2675-5.
- "The University of Iowa Economic Development Grow Iowa Values Fund Proposal: Fiscal Year 2011" (PDF). University of Iowa. pp. 13–16. Archived (PDF) from the original on 1 September 2016. Retrieved 1 September 2016.
- "Patents Assigned to Metabolic Technologies, Inc". Justia Patent.
As of March 2018, granted patents include: US8815280, US9259430, US9539224, US9707241, and US9770424.
External links
| Wikimedia Commons has media related to Beta-Hydroxy beta-methylbutyrate. |
- Beta-Hydroxyisovaleric acid at the US National Library of Medicine Medical Subject Headings (MeSH)