Lithium amide
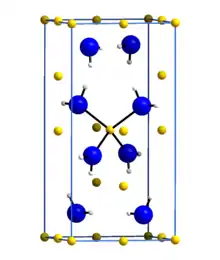
Lithium amide or lithium azanide is an inorganic compound with the chemical formula LiNH2. It is a white solid with a tetragonal crystal structure. Lithium amide can be made by treating lithium metal with liquid ammonia:[1]
- 2Li + 2NH3 → 2LiNH2 + H2
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Lithium amide | |
| Other names
Lithamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.062 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| LiNH 2 | |
| Molar mass | 22.96 g/mol |
| Appearance | white solid |
| Density | 1.178 g/cm3 |
| Melting point | 375 °C (707 °F; 648 K) |
| Boiling point | 430 °C (806 °F; 703 K) decomposes |
| reacts | |
| Solubility | slightly soluble in ethanol insoluble in ammonia |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
-182 kJ/mol |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Other lithium amides
The conjugate bases of amines are known as amides. Thus, a lithium amide may also refer to any compound in the class of the lithium salt of an amine. These compounds have the general form Li<NR2, with the chemical lithium amide itself as the parent structure. Common lithium amides include lithium diisopropylamide (LDA), lithium tetramethylpiperidide (LiTMP), and lithium hexamethyldisilazide (LiHMDS). They are produced by the reaction of Li metal with the appropriate amine:
- 2Li + 2R2NH → 2LiNR2 + H2
Lithium amides are very reactive compounds. Specifically, they are strong bases.
Examples
Lithium tetramethylpiperidide has been crystallised as a tetramer.[2] On the other hand, the lithium derivative of bis(1-phenylethyl)amine crystallises as a trimer:[3]
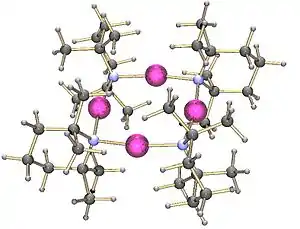 Tetrameric lithium tetramethylpiperidide |
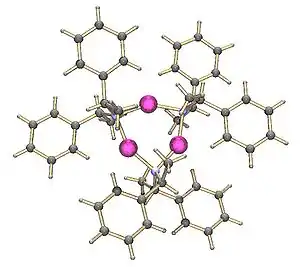 Trimeric lithium bis(1-phenylethyl)amide |
It is also possible to make mixed oligomers of metal alkoxides and amides.[4] These are related to the superbases which are mixtures of metal alkoxides and alkyls. The cyclic oligomers form when the nitrogen of the amide forms a sigma bond to a lithium while the nitrogen lone pair binds to another metal centre.
Other organolithium compounds (such as BuLi) are generally considered to exist in and function via high-order, aggregated species.
See also
References
- P. W. Schenk (1963). "Lithium amide". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. 1. NY,NY: Academic Press. p. 454.
- M.F. Lappert; M.J. Slade; A. Singh; J.L. Atwood; R.D. Rogers; R. Shakir (1983). "Structure and reactivity of sterically hindered lithium amides and their diethyl etherates: crystal and molecular structures of [Li{N(SiMe3)2}(OEt2)]2 and tetrakis(2,2,6,6-tetramethylpiperidinatolithium)". Journal of the American Chemical Society. 105 (2): 302–304. doi:10.1021/ja00340a031.
- D.R. Armstrong; K.W. Henderson; A.R. Kennedy; W.J. Kerr; F.S. Mair; J.H. Moir; P.H. Moran; R. Snaith (1999). "Structural studies of the chiral lithium amides [{PhC(H)Me}2NLi] and [PhCH2{PhC(H)Me}NLi·THF] derived from α-methylbenzylamine". Dalton Transactions: 4063–4068. doi:10.1039/A904725E.
- K.W. Henderson, D.S. Walther & P.G. Williard (1995). "Identification of a Unimetal Complex of Bases by 6Li NMR Spectroscopy and Single-Crystal Analysis". Journal of the American Chemical Society. 117 (33): 8680–8681. doi:10.1021/ja00138a030.
- Merck Index, 11th Edition, 5398.
