Lithium chloride
Lithium chloride is a chemical compound with the formula LiCl. The salt is a typical ionic compound (with certain covalent characters) , although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorides, such as extraordinary solubility in polar solvents (83.05 g/100 mL of water at 20 °C) and its hygroscopic properties.[5]
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Lithium chloride | |
| Systematic IUPAC name
Lithium(1+) chloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.028.375 |
| EC Number |
|
| MeSH | Lithium+chloride |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2056 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| LiCl | |
| Molar mass | 42.39 g·mol−1 |
| Appearance | white solid hygroscopic, sharp |
| Density | 2.068 g/cm3 |
| Melting point | 605–614 °C (1,121–1,137 °F; 878–887 K) |
| Boiling point | 1,382 °C (2,520 °F; 1,655 K) |
| 68.29 g/100 mL (0 °C) 74.48 g/100 mL (10 °C) 84.25 g/100 mL (25 °C) 88.7 g/100 mL (40 °C) 123.44 g/100 mL (100 °C)[1] | |
| Solubility | soluble in hydrazine, methylformamide, butanol, selenium(IV) oxychloride, propanol[1] |
| Solubility in methanol | 45.2 g/100 g (0 °C) 43.8 g/100 g (20 °C) 42.36 g/100 g (25 °C)[2] 44.6 g/100 g (60 °C)[1] |
| Solubility in ethanol | 14.42 g/100 g (0 °C) 24.28 g/100 g (20 °C) 25.1 g/100 g (30 °C) 23.46 g/100 g (60 °C)[2] |
| Solubility in formic acid | 26.6 g/100 g (18 °C) 27.5 g/100 g (25 °C)[1] |
| Solubility in acetone | 1.2 g/100 g (20 °C) 0.83 g/100 g (25 °C) 0.61 g/100 g (50 °C)[1] |
| Solubility in liquid ammonia | 0.54 g/100 g (-34 °C)[1] 3.02 g/100 g (25 °C) |
| Vapor pressure | 1 torr (785 °C) 10 torr (934 °C) 100 torr (1130 °C)[1] |
| −24.3·10−6 cm3/mol | |
Refractive index (nD) |
1.662 (24 °C) |
| Viscosity | 0.87 cP (807 °C)[1] |
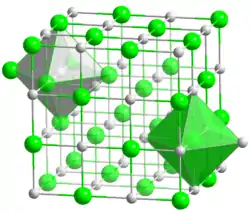
| Structure | |
| Octahedral | |
| Linear (gas) | |
| 7.13 D (gas) | |
| Thermochemistry | |
Heat capacity (C) |
48.03 J/mol·K[1] |
Std molar entropy (S |
59.31 J/mol·K[1] |
Std enthalpy of formation (ΔfH⦵298) |
-408.27 kJ/mol[1] |
Gibbs free energy (ΔfG˚) |
-384 kJ/mol[1] |
| Hazards | |
| Safety data sheet | See: data page ICSC 0711 |
| GHS pictograms |  [3] [3] |
| GHS Signal word | Warning |
| H302, H315, H319, H335[3] | |
| P261, P305+351+338[3] | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
526 mg/kg (oral, rat)[4] |
| Related compounds | |
Other anions |
Lithium fluoride Lithium bromide Lithium iodide Lithium astatide |
Other cations |
Sodium chloride Potassium chloride Rubidium chloride Caesium chloride Francium chloride |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chemical properties

The salt forms crystalline hydrates, unlike the other alkali metal chlorides.[6] Mono-, tri-, and pentahydrates are known.[7] The anhydrous salt can be regenerated by heating the hydrates. LiCl also absorbs up to four equivalents of ammonia/mol. As with any other ionic chloride, solutions of lithium chloride can serve as a source of chloride ion, e.g., forming a precipitate upon treatment with silver nitrate:
- LiCl + AgNO3 → AgCl + LiNO3
Preparation
Lithium chloride is produced by treatment of lithium carbonate with hydrochloric acid.[5] Anhydrous LiCl is prepared from the hydrate by heating with a stream of hydrogen chloride.
Uses
Commercial applications
Lithium chloride is mainly used for the production of lithium metal by electrolysis of a LiCl/KCl melt at 450 °C (842 °F). LiCl is also used as a brazing flux for aluminium in automobile parts. It is used as a desiccant for drying air streams.[5] In more specialized applications, lithium chloride finds some use in organic synthesis, e.g., as an additive in the Stille reaction. Also, in biochemical applications, it can be used to precipitate RNA from cellular extracts.[8]
Lithium chloride is also used as a flame colorant to produce dark red flames.
Niche uses
Lithium chloride is used as a relative humidity standard in the calibration of hygrometers. At 25 °C (77 °F) a saturated solution (45.8%) of the salt will yield an equilibrium relative humidity of 11.30%. Additionally, lithium chloride can itself be used as a hygrometer. This deliquescent salt forms a self-solution when exposed to air. The equilibrium LiCl concentration in the resulting solution is directly related to the relative humidity of the air. The percent relative humidity at 25 °C (77 °F) can be estimated, with minimal error in the range 10–30 °C (50–86 °F), from the following first order equation: RH=107.93-2.11C, where C is solution LiCl concentration, percent by mass.
Molten LiCl is used for the preparation of carbon nanotubes,[9] graphene[10] and lithium niobate.[11]
Lithium chloride has been shown to have strong acaricidal properties, being effective against Varroa destructor in populations of honey bees.[12]
Precautions
Lithium salts affect the central nervous system in a variety of ways. While the citrate, carbonate, and orotate salts are currently used to treat bipolar disorder, other lithium salts including the chloride were used in the past. For a short time in the 1940s lithium chloride was manufactured as a salt substitute, but this was prohibited after the toxic effects of the compound (tremors, fatigue, nausea) were recognized.[13][14][15]
References
- lithium chloride
- Seidell, Atherton; Linke, William F. (1952). Solubilities of Inorganic and Organic Compounds. Van Nostrand. Retrieved 2014-06-02.
- Sigma-Aldrich Co., Lithium chloride. Retrieved on 2014-05-09.
- ChemIDplus - 7447-41-8 - KWGKDLIKAYFUFQ-UHFFFAOYSA-M - Lithium chloride - Similar structures search, synonyms, formulas, resource links, and other chemical information
- Wietelmann, Ulrich; Bauer, Richard J. (2005). "Lithium and Lithium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_393.
- Holleman, A. F.; Wiberg, E. Inorganic Chemistry Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- Hönnerscheid Andreas; Nuss Jürgen; Mühle Claus; Jansen Martin (2003). "Die Kristallstrukturen der Monohydrate von Lithiumchlorid und Lithiumbromid". Zeitschrift für anorganische und allgemeine Chemie. 629 (2): 312–316. doi:10.1002/zaac.200390049.
- Cathala, G.; Savouret, J.; Mendez, B.; West, B. L.; Karin, M.; Martial, J. A.; Baxter, J. D. (1983). "A Method for Isolation of Intact, Translationally Active Ribonucleic Acid". DNA. 2 (4): 329–335. doi:10.1089/dna.1983.2.329. PMID 6198133.
- Kamali, Ali Reza; Fray, Derek J. (2014). "Towards large scale preparation of carbon nanostructures in molten LiCl". Carbon. 77: 835–845. doi:10.1016/j.carbon.2014.05.089.
- Kamali, Ali Reza; Fray, Derek J. (2015). "Large-scale preparation of graphene by high temperature insertion of hydrogen into graphite" (PDF). Nanoscale. 7 (26): 11310–11320. doi:10.1039/c5nr01132a. PMID 26053881.
- Kamali, Ali Reza; Fray, Derek J. (2014). "Preparation of lithium niobate particles via reactive molten salt synthesis method". Ceramics International. 40: 1835–1841. doi:10.1016/j.ceramint.2013.07.085.
- Ziegelmann, Bettina; Abele, Elisabeth (January 12, 2018). "Lithium chloride effectively kills the honey bee parasite Varroa destructor by a systemic mode of action". Scientific Reports. 8 (1): 683. Bibcode:2018NatSR...8..683Z. doi:10.1038/s41598-017-19137-5. PMC 5766531. PMID 29330449.
- Talbott J. H. (1950). "Use of lithium salts as a substitute for sodium chloride". Arch Intern Med. 85 (1): 1–10. doi:10.1001/archinte.1950.00230070023001. PMID 15398859.
- L. J. Stone; M. luton; J. Gilroy (1949). "Lithium Chloride as a Substitute for Sodium Chloride in the Diet". Journal of the American Medical Association. 139 (11): 688–692. doi:10.1001/jama.1949.02900280004002. PMID 18128981.
- "Case of trie Substitute Salt". Time. 28 February 1949.
- Handbook of Chemistry and Physics, 71st edition, CRC Press, Ann Arbor, Michigan, 1990.
- N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997.
- R. Vatassery, titration analysis of LiCl, sat'd in Ethanol by AgNO3 to precipitate AgCl(s). EP of this titration gives %Cl by mass.
- H. Nechamkin, The Chemistry of the Elements, McGraw-Hill, New York, 1968.
