Mangrove tree distribution
Global mangrove distributions have fluctuated throughout human and geological history. The area covered by mangroves is influenced by a complex interaction between land position, rainfall hydrology, sea level, sedimentation, subsidence, storms and pest-predator relationships[1][2]). In the last 50 years, human activities have strongly affected mangrove distributions, resulting in declines or expansions of worldwide mangrove area. Mangroves provide several important ‘free services’ including coastal stabilization, juvenile fish habitats, and the filtration of sediment and nutrients[3][4][5]). Mangrove loss has important implications for coastal ecological systems and human communities dependent on healthy mangrove ecosystems. This English Wikipedia page presents an overview of global Mangrove Forest biome trends in mangrove ecoregions distribution, as well as the cause of such changes.
As of 2012, mangroves are found in 105 nations globally.[6] Although distributed across 105 nations, the top 10 mangrove holding nations contain approximately 52% of the global mangrove stock with Indonesia alone containing between 26% and 29% of the entire global mangrove stock.[6] The largest continuous area of mangrove forest is likely in-and-around the Sundarbans National Park in India and the Sundarbans Mangrove Forests in Bangladesh,[7] which are both recognized by UNESCO as World Heritage Sites.[8] Although existing almost exclusively in the tropics and near-tropics, warm ocean currents support mangrove forests as far north as Walsingham Nature Reserve (Idwal Hughes Nature Reserve) in Bermuda and as far south as Snake Island, Australia.[6]
Top 20 Mangrove Holdings Nations in 2012.[6]
| Rank | Country Name | Treecover in Mangrove Forests | Treecover in Wider Mangrove Biome |
| km2 | km2 | ||
| 1 | Indonesia | 23,324.29 | 44,038.77 |
| 2 | Brazil | 7,674.94 | 17,685.60 |
| 3 | Malaysia | 4,725.84 | 8,231.09 |
| 4 | Papua New Guinea | 4,172.29 | 5,677.16 |
| 5 | Australia | 3,316.21 | 3,251.24 |
| 6 | Mexico | 2,991.83 | 6,203.92 |
| 7 | Myanmar | 2,557.45 | 3,856.09 |
| 8 | Nigeria | 2,653.99 | 6,919.28 |
| 9 | Venezuela | 2,403.83 | 7,539.12 |
| 10 | Philippines | 2,064.24 | 2,089.24 |
| 11 | Thailand | 1,886.33 | 4,196.97 |
| 12 | Bangladesh | 1,772.98 | 2,314.56 |
| 13 | Colombia | 1,671.86 | 6,274.70 |
| 14 | Cuba | 1,633.46 | 2,454.10 |
| 15 | United States | 1,568.60 | 1,585.06 |
| 16 | Panama | 1,323.94 | 2,708.21 |
| 17 | Mozambique | 1,223.67 | 2,677.27 |
| 18 | Cameroon | 1,112.76 | 1,332.16 |
| 19 | Gabon | 1,082.11 | 3,882.95 |
| 20 | Ecuador | 935.74 | 1,945.27 |
Mangrove declines
Global trends
In the last five decades, worldwide mangrove area has fallen across all regions.[3][9][10] Different data sources or survey methods make estimates more problematic, as many nations have high variations of mangrove change.[3][9][10][11]
Percentage of original area lost per year
- Asia: 1.52%
- Africa: 1.25%
- Australia: 1.99%
- Americas: 3.62%
- The World: 2.07%
(based on data from)[3] (Annual loss rates calculated from the mean number of years between original area and present area for each region: 24, 25, 7, 11, and 17 years for Asia, Africa, Australia, the Americas, and the world, respectively.)
From 2000 to 2012, the global mangrove deforestation rate was between 0.16% and 0.39% annually but as high as 3.58% to 8.08% in Southeast Asia.[6] The most recent and comprehensive global assessment of mangrove distribution was conducted by Hamilton and Casey (2016) and provides a high-resolution global database of mangrove loss at the 1 arc-second level at annual intervals from 2000 to 2012 with estimates for 2013 and 2014.[6] In the 1980s and 1990s the greatest amount of loss occurred, while in the period of 2000 to 2005 the rate has fallen significantly across all regions.[10] Some projections estimate that worldwide mangrove area will decline by a further 25% by 2025, particularly in developing nations.[12] It is estimated that since 1970, 28% of mangrove lost has been directly displaced by commercial aquaculture.[13]
Role of perception
People’s perception of mangrove ecosystems has been instrumental in the loss of mangroves. Commonly, they have been undervalued and considered ‘wastelands’ with low productivity (,[10][12][14]). Concerns for the fate of mangroves have historically been restricted to scientific communities, with little transfer of knowledge to local communities and governments.[3] Mangroves are a common pool resource, creating difficulties in enforcing restrictions on exploitative activities.[15] More recently, perceptions have shifted to a broader acknowledgment within communities and governments of the value of mangroves in coastal ecosystems and local communities. The declining rate of mangrove loss since 2000 across all regions is indicative of this, bringing forth an increasing number of conservation projects and legislation.[10]
Causes of decline
A variety of factors have led to the declining trends in mangrove distribution (refer to table fig of causes). These factors are predominantly anthropogenic in origin, as mangrove destruction is positively correlated to human population density.[9] Up to 35% of mangrove forests worldwide have been lost since 1980, reflecting the increased pressure from high population densities residing at the coast.[3][9][10] Mangrove losses to climate change are considered a probable long term threat to future distribution.[9][12] Despite a wide variety of factors linked to mangrove loss (insert figure of causes), the main drivers of recent (i.e. after 1980) mangrove destruction are linked to four main activities: urban sprawl, tourism, agriculture and aquaculture.[3][9][10][16] The causes of declines in mangrove areas vary between regions. In Asia, the Caribbean and Latin America, aquaculture and tourism development are the greatest threat.[10] In Oceania, tourism development is the greatest threat while in Africa, conversion of mangroves for agricultural and urban development is most apparent.[10]
Aquaculture – This activity is considered the greatest contributor to worldwide mangrove loss.[11][12][13] Since the 1980s, shrimp aquaculture dramatically increased, replacing mangroves with ponds and degrading surrounding areas with associated pollution.[3][4][10] Aquaculture has dramatically affected some regions. For example, 50–80% of mangroves were lost in Asia in the past decades.[16] Asia, the Caribbean and Latin America have been most affected by aquaculture[10]
.jpg.webp)
Urban expansion – As population density increases at the coast, areas of mangrove forest have been replaced to provide urban and industrial lands.[10]
Tourism – infrastructure supporting tourism often requires the reclamation of substantial tracts of mangrove forests.[10] In the Americas and Oceania, tourism is a major driver of mangrove loss. Where tourism is a main contributor to an economy (such as Pacific Island nations), construction of resorts and related infrastructures have reduced mangrove area in past decades.[10]
Agriculture – Conversion of mangrove forests for agriculture has occurred across all regions historically, freeing up land for activities such as rice or salt production.[10][16]
Charcoal: As of 2019 in Myanmar, the cutting down of mangroves to turn into charcoal for sale in China and Thailand continues unabated.[17]
Mangrove loss case study: shrimp aquaculture in South-east Asia
In many South-east Asian nations, shrimp aquaculture has been instrumental in mangrove loss, particularly in the 1980s and 1990s.[3][4][10] Due to high economic returns, shrimp farming was promoted to improve economic conditions in many countries.[4][11] The social and economic benefits of shrimp farming are substantial.[4] Shrimp farms are located close to the coast to reduce costs by using tidal energy and short canal lengths to the coast.[4] Consequently, vast tracts of mangrove forest have been replaced by numerous ponds. Compounding mangrove losses is the short life span of individual ponds (5–10 years), imposing a shifting cultivation pattern to shrimp farming.[3]
After 1980, shrimp farming became far more intensive, with high productivity (per unit area) ponds proliferating across the landscape.[4] Dramatic reductions in mangrove forests were reported with shrimp pond intensification. In Thailand, mangrove forests literally halved between 1975–1993[18] The impact of such losses has been linked to the disruption of ecological and economic functioning of mangroves.[11] For example, loss of income for local Thai fishermen has been reported due to reduced catches from the degradation of juvenile fish habitat.[11][18] Some consider that the number of shrimp farms in South-east Asia peaked in the late 1990s, with the rate of mangrove loss declining accordingly.[18] This decline is in part due to increased conservation and restoration projects, and improved management practices.
Global climate change and mangrove loss
Increases in temperature, CO
2, precipitation, storms, and sea level are likely to threaten mangroves in the future.[12] Sea level rise is considered the greatest climate change related threat to mangrove regions.[9][12] The natural ability of mangroves to ‘keep up’ with rising sea level through peat or sediment accumulation could be exceeded, leading to mangrove die back.[19]Low island mangroves are most at risk, as demonstrated in Bermuda, where sea level rise has exceeded peat accumulation rates periodically, resulting in landward die back of mangrove stands.[19] Climate change may reduce global mangrove area by 10–15%, but it is a long term, less significance threat to the current 1–2% annual loss from human activities.[9]
Mangrove expansions
Global trends
Several nations have experienced an expansion of their mangrove area. These expansions include human activities promoting seaward or landward expansion from climatic or other local factors, as well as conservation and reforestation programmes.[5][10][16][20][21] Globally, this stability and expansion is far outweighed by the magnitude of mangrove loss from human activities.
Seaward expansion and human activity
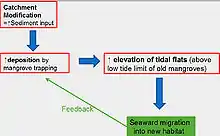
Seaward mangrove expansion is considered a natural response to high inputs of sediment and nutrients from human activity in adjacent catchments;.[5][20][22] Mangroves naturally encourage sediment deposition by slowing currents and attenuating waves. However, with high sediment inputs, the elevation of intertidal flats can rise above the low tide limit of the original mangrove forest, resulting in a seaward expansion of new mangrove habitat.[5] Once new mangroves establish, they too begin to trap more sediment, raising the tidal flat elevation and generating more mangrove habitat.[5] Seaward expansions are likely in regions with modified catchments (i.e. deforestation or urban development) with high topography where high sediments loads are delivered to mangroves at the coast.[20] Extremely high rates of sediment input can exceed tolerance thresholds and induce mangrove die backs.[14] Excess sedimentation is the primary control, but nutrients (i.e. from agricultural runoff) can enhance mangrove expansion, particularly when combined with high levels of sedimentation.[22] Seaward expansions highlight the interconnectivity of mangrove ecosystems to adjacent catchments and the need to consider catchment alteration when managing mangroves.
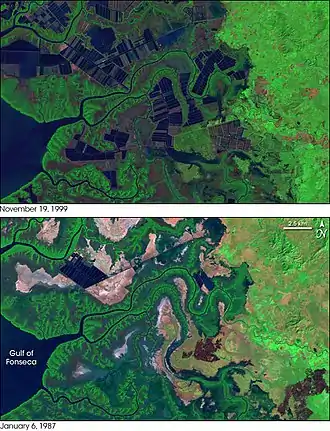
Case study: New Zealand mangroves
In New Zealand (NZ), temperate mangroves (Avicennia marina) are common in estuaries, harbours and tidal creeks north of 38° latitude.[5] Following European colonization, mangrove area declined, but since the 1970s rapid, ongoing seaward expansion has occurred in many areas.[5][20][22][23] For example, in the last 50 years mangrove area has expanded 120% in Tuaranga Harbour.[23] Such expansion is strongly correlated with elevated rates of sediment deposition. Catchment activities such as deforestation and more recently, urban development have been blamed for the expansion.[5][20][22][23] Considerable debate over whether to protect or remove NZ mangroves exist. Proponents for protection cite the ecological and coastal protection values of mangrove stands, while removal advocates aim to restore recreational values and prevent encroachment into non-mangrove ecosystems.[20] A shift in the management strategy of regional councils from protection to mangrove ‘management’ has occurred recently[20][24] Removals may take place when human amenities or non-mangrove habitats are deemed threatened by mangrove encroachment.[20][24] Some researchers caution comparisons being made between NZ and tropical mangroves, suggesting NZ mangroves are unique in their ecology and require a tailored management approach.[20]
Landward expansion
In contrast to seaward expansions in NZ, landward expansions are documented in a number of regions (Thailand, Australia, and Hawaii).[1][20][21][25] The cause of landward expansions is not always clear. In Eastern Australia expansions have replaced salt marshes with various suggestions for the cause of landward expansion (increased rainfall, changes in hydrology, to local subsidence).[1][20][21] Expansions in Hawaii are thought to be the result of invasions by exotic mangrove species.[25]
Expansion through conservation and restoration
More recently, awareness of the economic, social, and ecological values of mangroves have led to an increase in the number of initiatives to protect and restore mangrove areas.[3][10] Broader recognition of the connection of mangroves to coastal food chains, coastal protection and socio-economic welfare has driven recent conservation.[10] For example, Bangladesh has undertaken extensive coastal afforesation (mass plantings) projects since 1966, leading to an increase in mangrove area in recent decades;[10][26] 2005;.[9] Many nations with mangroves have signed on to the 1971 Ramsar Convention on wetlands in the last two decades, making a commitment to wetland preservation.[27] However, the adoption of protective legislation is not evenly spread. For instance, community initiatives to protect mangroves are common in Africa but little legislation exists compared to other regions, such as Asia.[10]
Poleward Expansion
Poleward expansion of mangrove species has been observed on many coast lines in the world including US East and West coast, as well as Australia. This is arguably related to climate change. Average lowest temperatures have been increasing due to climate change, and this trend allows the survival of propagules during winter and germinate in warmer seasons.[28][29] In areas that were too cold for mangrove species to grow, mangrove's survival could results in invasive species. In New South Wales in Australia, area coverage of salt marsh has declined due to poleward expansion of mangrove species.[30]
Challenges, limitations and future suggestions
Much attention has been given to mangroves by the scientific community, as well as a largely universal acknowledgement among communities and governments of the ecological and economic values.[16] Despite this awareness, global mangrove area continues to decline through human activities. A lack of communication between biologists, effected residents, and governments could partly account for this.[16] Calls have been made for greater collaboration between international researchers and residents of affected nations. Efforts to assess changes in worldwide mangrove distribution have been hindered by a lack of long term data, or limited to no data at all for some nations.[9][16] Without accurate data on distributions, an accurate forecast of mangrove loss or expansion becomes problematic. This in turn may hinder the progress of conservation projects. Despite large volumes of scientific literature on mangroves, many uncertainties still exist. These include how mangroves will respond to increases in greenhouse gases and sea level, sustainable yields for silviculture, the difference in ecological functioning between tropical and temperate species, and why within the same species we get seaward and landward expansions.[5][9] Obtaining this knowledge will help nations to better predict future mangrove behavior, and administer appropriate management policies.
Summary
Mangrove loss has been a worldwide trend for the last 50 years due primarily to anthropogenic activities that compete for land area. Expansions in mangrove area do occur, but have a limited ability to offset the extensive losses. Recently the rate of loss has been declining due to a greater awareness of mangrove values, with legislation and conservation projects becoming more commonplace. It can only be hoped that this declining rate of loss continues, allowing future generations to appreciate the many benefits of mangroves.
See also
|
References
- Eslami-Andargoli, L., Dale, P. et al. (2009). "Mangrove expansion and rainfall patterns in Moreton Bay, Southeast Queensland, Australia." Estuarine, Coastal and Shelf Science 85(2): 292–298.
- "McLeod and Salm 2006" McLeod, E. and R. V. Salm (2006). Managing mangroves for resilience to climate change. Gland, Switzerland, The World Conservation Union (IUCN) 2.
- Valiela, I., Bowen, J. L. & York, J. K. 2001. Mangrove forests: one of the world's threatened major tropical environments. BioScience, 51, 807–815.
- Dierberg, F. E. & Kiattisimkul, W. 1996. Issues, impacts, and implications of shrimp aquaculture in Thailand. Environmental Management, 20, 649–666.
- Morrisey, D., Beard, C., Morrison, M., Craggs, R. & Lowe, M. 2007. The New Zealand Mangrove: Review of the Current State Of Knowledge. Auckland Regional Council.
- Hamilton, Stuart E.; Casey, Daniel (2016-06-01). "Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21)". Global Ecology and Biogeography. 25 (6): 729–738. arXiv:1412.0722. doi:10.1111/geb.12449. ISSN 1466-8238. S2CID 55999275.
- Giri, C.; Ochieng (2013). "Conservation; climate; marine-and-coastal; remote-sensing; sustainability". Global Mangrove Forests Distribution, v1: Land Use and Land Cover (LULC) | SEDAC. Palisades, NY: NASA Socioeconomic Data and Applications Center (SEDAC). doi:10.7927/h4j67dw8.
- Centre, UNESCO World Heritage. "UNESCO World Heritage Centre – World Heritage List". whc.unesco.org. Retrieved 2016-10-21.
- Alongi, D. M. 2002. Present state and future of the world's mangrove forests. Environmental Conservation, 29, 331–349.
- FAO 2007. The World's Mangroves 1980–2005. FAO Forestry Paper. Rome: Food and Agriculture Organisation of the United Nations (FAO) no.153
- Barbier, E. & Sathirathai, S. 2004. Shrimp farming and mangrove loss in Thailand, Cheltenham, Edward Elgar Publishing.
- McLeod, E. & Salm, R. V. 2006. Managing mangroves for resilience to climate change. Gland, Switzerland: The World Conservation Union (IUCN)
- Hamilton, Stuart (2013-04-01). "Assessing the Role of Commercial Aquaculture in Displacing Mangrove Forest". Bulletin of Marine Science. 89 (2): 585–601. doi:10.5343/bms.2012.1069.
- Krauss, K. W., Lovelock, C. E., McKee, K. L., Lopez-Hoffman, L., Ewe, S. M. L. & Sousa, W. P. 2008. Environmental drivers in mangrove establishment and early development: A review. Aquatic Botany, 89, 105–127.
- Idrus, M. R. 2009. Hard habits to break : investigating coastal resource utilisations and management systems in Sulawesi, Indonesia : a thesis submitted in partial of the requirements for the degree of Doctor of Philosophy in Environmental Science at the University of Canterbury.
- Farnsworth, E. J. & Ellison, A. M. 1997. The global conservation status of mangroves. Ambio, 328–334.
- Yan, Wudan (2019-04-18). "llegal charcoal trade threatens Myanmar's remaining mangroves". Mongabay. Retrieved 2019-04-20.
- Aksornkoae, S. & Tokrishna, R. 2004. Overview of shrimp farming and mangrove loss in Thailand. In: Barbier, E. & Sathirathai, S. (eds.) Shrimp farming and mangrove loss in Thailand. Cheltenham Edward Elgar Publishing.
- Ellison, J. C. 1993. Mangrove retreat with rising sea-level, Bermuda. Estuarine, Coastal and Shelf Science, 37, 75–87.
- Harty, C. 2009. Mangrove planning and management in New Zealand and South East Australia – A reflection on approaches. Ocean and Coastal Management, 52, 278–286.
- Saintilan, N. & Wilton, K. 2001. Changes in the distribution of mangroves and saltmarshes in Jervis Bay, Australia. Wetlands Ecology and Management, 9, 409–420.
- Lovelock, C. E., Feller, I. C., Ellis, J., Schwarz, A. M., Hancock, N., Nichols, P. & Sorrell, B. 2007. Mangrove growth in New Zealand estuaries: The role of nutrient enrichment at sites with contrasting rates of sedimentation.
- Park, S. 2004. Aspects of Mangrove Distribution and Abundance in Tauranga Harbour. Whakatane: Environment Bay of Plenty, 2004/16.
- De Luca, S. 2009. Mangrove Management – Balancing Values. Coastal News, NZ no. 42.
- Chimner, R. A., Fry, B., Kaneshiro, M. Y. & Cormier, N. 2006. Current Extent and Historical Expansion of Introduced Mangroves on O'ahu, Hawai'i1. Pacific Science, 60, 377–383
- Saenger, N. A. 1993. Land from the sea: the mangrove afforestation program of Bangladesh. Ocean & Coastal Management, 20, 23–39
- Ramsar. 1971. Ramsar Convention on Wetlands [Online]. Available: http://www.ramsar.org/cda/en/ramsar-about-about-ramsar/main/ramsar/1-36%5E7687_4000_0__ [Accessed April 2010].
- Cavanaugh, K. C.; Kellner, J. R.; Forde, A. J.; Gruner, D. S.; Parker, J. D.; Rodriguez, W.; Feller, I. C. (2013-12-30). "Poleward expansion of mangroves is a threshold response to decreased frequency of extreme cold events". Proceedings of the National Academy of Sciences. 111 (2): 723–727. doi:10.1073/pnas.1315800111. ISSN 0027-8424. PMC 3896164. PMID 24379379.
- Cavanaugh, Kyle C.; Parker, John D.; Cook-Patton, Susan C.; Feller, Ilka C.; Williams, A. Park; Kellner, James R. (2015-02-06). "Integrating physiological threshold experiments with climate modeling to project mangrove species' range expansion". Global Change Biology. 21 (5): 1928–1938. doi:10.1111/gcb.12843. ISSN 1354-1013. PMID 25558057.
- Saintilan, Neil; Wilson, Nicholas C.; Rogers, Kerrylee; Rajkaran, Anusha; Krauss, Ken W. (2013-11-11). "Mangrove expansion and salt marsh decline at mangrove poleward limits". Global Change Biology. 20 (1): 147–157. doi:10.1111/gcb.12341. ISSN 1354-1013. PMID 23907934.