Mangrove
A mangrove is a shrub or small tree that grows in coastal saline or brackish water. The term is also used for tropical coastal vegetation consisting of such species. Mangroves occur worldwide in the tropics and subtropics, mainly between latitudes 25° N and 25° S. The total mangrove forest area of the world in 2000 was 137,800 square kilometres (53,200 sq mi), spanning 118 countries and territories.[1]

Mangroves are salt-tolerant trees, also called halophytes, and are adapted to life in harsh coastal conditions. They contain a complex salt filtration system and complex root system to cope with salt water immersion and wave action. They are adapted to the low oxygen conditions of waterlogged mud.[2]
The word is used in at least three senses: (1) most broadly to refer to the habitat and entire plant assemblage or mangal,[3] for which the terms mangrove forest biome, and mangrove swamp are also used, (2) to refer to all trees and large shrubs in a mangrove swamp, and (3) narrowly to refer just to "true" mangrove trees of the genus Rhizophora of the family Rhizophoraceae.
The mangrove biome, or mangal, is a distinct saline woodland or shrubland habitat characterized by depositional coastal environments, where fine sediments (often with high organic content) collect in areas protected from high-energy wave action. The saline conditions tolerated by various mangrove species range from brackish water, through pure seawater (3 to 4% salinity), to water concentrated by evaporation to over twice the salinity of ocean seawater (up to 9% salinity).[4]
Etymology
The term "mangrove" comes to English from Spanish (perhaps by way of Portuguese), and is likely to originate from Guarani. It was earlier "mangrow" (from Portuguese mangue or Spanish mangle), but this word was corrupted via folk etymology influence of the word "grove". It could possibly also come from Spanish directly from Taíno (mangle).[5]
Ecology
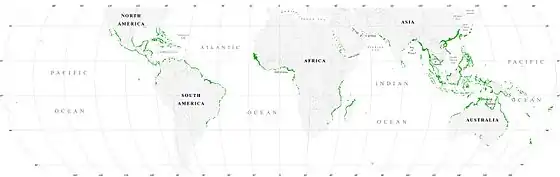

Mangrove swamps (mangals) are found in tropical and subtropical tidal areas. Areas where mangroves occur include estuaries and marine shorelines.[6]
The intertidal existence to which these trees are adapted represents the major limitation to the number of species able to thrive in their habitat. High tide brings in salt water, and when the tide recedes, solar evaporation of the seawater in the soil leads to further increases in salinity. The return of tide can flush out these soils, bringing them back to salinity levels comparable to that of seawater.
At low tide, organisms are also exposed to increases in temperature and reduced moisture before being then cooled and flooded by the tide. Thus, for a plant to survive in this environment, it must tolerate broad ranges of salinity, temperature, and moisture, as well as a number of other key environmental factors—thus only a select few species make up the mangrove tree community.
About 110 species are considered "mangroves", in the sense of being a tree that grows in such a saline swamp,[6] though only a few are from the mangrove plant genus, Rhizophora. However, a given mangrove swamp typically features only a small number of tree species. It is not uncommon for a mangrove forest in the Caribbean to feature only three or four tree species. For comparison, the tropical rainforest biome contains thousands of tree species, but this is not to say mangrove forests lack diversity. Though the trees themselves are few in species, the ecosystem that these trees create provides a home (habitat) for a great variety of other species, including as many as 174 species of marine megafauna.[7]
Mangrove plants require a number of physiological adaptations to overcome the problems of low environmental oxygen levels, high salinity and frequent tidal flooding. Each species has its own solutions to these problems; this may be the primary reason why, on some shorelines, mangrove tree species show distinct zonation. Small environmental variations within a mangal may lead to greatly differing methods for coping with the environment. Therefore, the mix of species is partly determined by the tolerances of individual species to physical conditions, such as tidal flooding and salinity, but may also be influenced by other factors, such as crabs preying on plant seedlings.
Once established, mangrove roots provide an oyster habitat and slow water flow, thereby enhancing sediment deposition in areas where it is already occurring. The fine, anoxic sediments under mangroves act as sinks for a variety of heavy (trace) metals which colloidal particles in the sediments have scavenged from the water. Mangrove removal disturbs these underlying sediments, often creating problems of trace metal contamination of seawater and organisms of the area.
Mangrove swamps protect coastal areas from erosion, storm surge (especially during hurricanes), and tsunamis.[8][9][10] The mangroves' massive root systems are efficient at dissipating wave energy.[11] Likewise, they slow down tidal water enough so its sediment is deposited as the tide comes in, leaving all except fine particles when the tide ebbs.[12] In this way, mangroves build their own environments.[8] Because of the uniqueness of mangrove ecosystems and the protection against erosion they provide, they are often the object of conservation programs, including national biodiversity action plans.[9]
Mangrove swamps' effectiveness in terms of erosion control can sometimes be overstated. Wave energy is typically low in areas where mangroves grow,[13] so their effect on erosion is measured over long periods.[11] Their capacity to limit high-energy wave erosion is in relation to events such as storm surges and tsunamis.[14]
The unique ecosystem found in the intricate mesh of mangrove roots offers a quiet marine region for young organisms.[15] In areas where roots are permanently submerged, the organisms they host include algae, barnacles, oysters, sponges, and bryozoans, which all require a hard surface for anchoring while they filter feed. Shrimps and mud lobsters use the muddy bottoms as their home.[16] Mangrove crabs munch on the mangrove leaves, adding nutrients to the mangal mud for other bottom feeders.[17] In at least some cases, export of carbon fixed in mangroves is important in coastal food webs.
Mangrove plantations in Vietnam, Thailand, Philippines and India host several commercially important species of fishes and crustaceans. Despite restoration efforts, developers and others have removed over half of the world's mangroves in recent times.
Mangrove forests can decay into peat deposits because of fungal and bacterial processes as well as by the action of termites. It becomes peat in good geochemical, sedimentary and tectonic conditions.[18] The nature of these deposits depends on the environment and the types of mangrove involved. In Puerto Rico the red (Rhizophora mangle), white (Laguncularia racemosa) and black (Avicennia germinans) mangroves occupy different ecological niches and have slightly different chemical compositions so the carbon content varies between the species as well between the different tissues of the plant e.g. leaf matter vs. roots.[18]
In Puerto Rico, there is a clear succession of these three trees from the lower elevations which are dominated by red mangroves to farther inland with a higher concentration of white mangroves.[18] Mangrove forests are an important part of the cycling and storage of carbon in tropical coastal ecosystems.[18] Using this it is possible to attempt to reconstruct the environment and investigate changes to the coastal ecosystem for thousands of years by using sediment cores.[19] However, an additional complication is the imported marine organic matter that also gets deposited in the sediment due to tidal flushing of mangrove forests.[18]
In order to understand peat formation by mangroves, it is important to understand the conditions they grew in, and how they decayed. Termites are an important part of this decay, and so an understanding of their action on the organic matter is crucial to the chemical stabilization of mangrove peats.[18]
Mangroves are an important source of blue carbon. Globally, mangroves stored 4.19 Pg of carbon in 2012.[20] Two percent of global mangrove carbon was lost between 2000 and 2012, equivalent to a maximum potential of 316,996,250 t of CO2 emissions.[20]
Mangroves are shown to provide measurable economic protections to coastal communities to tropical storm impacted communities globally.[21]
Biology
.jpg.webp)


.jpg.webp)
Of the recognized 110 mangrove species, only about 54 species in 20 genera from 16 families constitute the "true mangroves", species that occur almost exclusively in mangrove habitats.[3] Demonstrating convergent evolution, many of these species found similar solutions to the tropical conditions of variable salinity, tidal range (inundation), anaerobic soils and intense sunlight. Plant biodiversity is generally low in a given mangrove.[6] The greatest biodiversity occurs in the mangal of New Guinea, Indonesia and Malaysia.[22]
Adaptations to low oxygen
Red mangroves, which can survive in the most inundated areas, prop themselves above the water level with stilt roots and can then absorb air through pores in their bark (lenticels). Black mangroves live on higher ground and make many pneumatophores (specialised root-like structures which stick up out of the soil like straws for breathing) which are also covered in lenticels.
These "breathing tubes" typically reach heights of up to 30 cm, and in some species, over 3 m. The four types of pneumatophores are stilt or prop type, snorkel or peg type, knee type, and ribbon or plank type. Knee and ribbon types may be combined with buttress roots at the base of the tree. The roots also contain wide aerenchyma to facilitate transport within the plants.
Nutrient uptake
Because the soil is perpetually waterlogged, little free oxygen is available. Anaerobic bacteria liberate nitrogen gas, soluble ferrum (iron), inorganic phosphates, sulfides and methane, which make the soil much less nutritious. Pneumatophores (aerial roots) allow mangroves to absorb gases directly from the atmosphere, and other nutrients such as iron, from the inhospitable soil. Mangroves store gases directly inside the roots, processing them even when the roots are submerged during high tide.
Limiting salt intake
Red mangroves exclude salt by having significantly impermeable roots which are highly suberised (impregnated with suberin), acting as an ultra-filtration mechanism to exclude sodium salts from the rest of the plant. Analysis of water inside mangroves has shown 90% to 97% of salt has been excluded at the roots. In a frequently cited concept that has become known as the "sacrificial leaf", salt which does accumulate in the shoot (sprout) then concentrates in old leaves, which the plant then sheds. However, recent research suggests the older, yellowing leaves have no more measurable salt content than the other, greener leaves.[23] Red mangroves can also store salt in cell vacuoles. White and grey mangroves can secrete salts directly; they have two salt glands at each leaf base (correlating with their name—they are covered in white salt crystals).
Limiting water loss
Because of the limited fresh water available in salty intertidal soils, mangroves limit the amount of water they lose through their leaves. They can restrict the opening of their stomata (pores on the leaf surfaces, which exchange carbon dioxide gas and water vapour during photosynthesis). They also vary the orientation of their leaves to avoid the harsh midday sun and so reduce evaporation from the leaves. Anthony Calfo, a noted aquarium author, observed anecdotally a red mangrove in captivity only grows if its leaves are misted with fresh water several times a week, simulating frequent tropical rainstorms.[24]
Increasing survival of offspring
In this harsh environment, mangroves have evolved a special mechanism to help their offspring survive. Mangrove seeds are buoyant and are therefore suited to water dispersal. Unlike most plants, whose seeds germinate in soil, many mangroves (e.g. red mangrove) are viviparous, meaning their seeds germinate while still attached to the parent tree. Once germinated, the seedling grows either within the fruit (e.g. Aegialitis, Avicennia and Aegiceras), or out through the fruit (e.g. Rhizophora, Ceriops, Bruguiera and Nypa) to form a propagule (a ready-to-go seedling) which can produce its own food via photosynthesis.
The mature propagule then drops into the water, which can transport it great distances. Propagules can survive desiccation and remain dormant for over a year before arriving in a suitable environment. Once a propagule is ready to root, its density changes so the elongated shape now floats vertically rather than horizontally. In this position, it is more likely to lodge in the mud and root. If it does not root, it can alter its density and drift again in search of more favorable conditions.
Taxonomy and evolution
The following listing (modified from Tomlinson, 1986) gives the number of species of mangroves in each listed plant genus and family. Mangrove environments in the Eastern Hemisphere harbor six times as many species of trees and shrubs as do mangroves in the New World. Genetic divergence of mangrove lineages from terrestrial relatives, in combination with fossil evidence, suggests mangrove diversity is limited by evolutionary transition into the stressful marine environment, and the number of mangrove lineages has increased steadily over the Tertiary with little global extinction.[25]
Major components
| Family | Genus, number of species | Common name |
|---|---|---|
| Acanthaceae, Avicenniaceae or Verbenaceae (family allocation disputed) |
Avicennia, 9 | Black mangrove |
| Combretaceae | Conocarpus, 1 (C. erectus); Laguncularia, 1 (L. racemosa); Lumnitzera, 3 | Buttonwood, white mangrove |
| Arecaceae | Nypa, 1 (N. fruticans) | Mangrove palm |
| Rhizophoraceae | Bruguiera, 7; Ceriops, 5; Kandelia, 2; Rhizophora, 8 | Red mangrove |
| Lythraceae | Sonneratia, 5 | Mangrove apple |
Minor components
| Family | Genus, number of species |
|---|---|
| Acanthaceae | Acanthus, 2 (A. ebracteatus, A. ilicifolius); Bravaisia, 2 (B. berlandieriana, B. integerrima) |
| Arecaceae | Phoenix, 1 (P. paludosa) |
| Cyperaceae | Fimbristylis, 1 |
| Euphorbiaceae | Excoecaria, 2 |
| Lecythidaceae | Barringtonia, 6 |
| Lythraceae | Pemphis, 2 |
| Malvaceae | Camptostemon, 2 (C. philippinensis, C. schultzii); Heritiera, 3 (H. fomes, H. globosa, H. littoralis) |
| Meliaceae | Xylocarpus, 2 (X. granatum, X. moluccensis) |
| Myrtaceae | Osbornia, 1 (O. octodonta) |
| Pellicieraceae | Pelliciera, 1 (P. rhizophorae) |
| Plumbaginaceae | Aegialitis, 2 (A. annulata, A. rotundifolia) |
| Primulaceae | Aegiceras, 2 (A. corniculatum, A. floridum) |
| Pteridaceae | Acrostichum, 3 (A. aureum, A. danaeifolium, A. speciosum) |
| Rubiaceae | Scyphiphora, 1 (S. hydrophyllacea) |
Geographical regions
Mangroves can be found in over one hundred countries and territories in the tropical and subtropical regions of the world. The largest percentage of mangroves is found between the 5° N and 5° S latitudes. Approximately 75% of world's mangroves are found in just 15 countries. Asia has the largest amount (42%) of the world's mangroves, followed by Africa (21%), Northern, Central America and the Caribbean (15%), Oceania (12%), and South America (11%).[1] The latest remotely-sensed global synthesis estimates global mangrove forest area post-2000 as 141,333 km2 ±6% (CI 0.9, n = 4).[26]
Top 20 mangrove habitat countries

| Rank | Country | Tree cover (km2) in mangrove forests |
Tree cover (km2) in mangrove biome |
|---|---|---|---|
| 1 | 23,143 | 42,278 | |
| 2 | 7,663 | 17,287 | |
| 3 | 4,691 | 7,616 | |
| 4 | 4,169 | 6,236 | |
| 5 | 3,315 | 3,314 | |
| 6 | 2,985 | 6,036 | |
| 7 | 2,653 | 6,908 | |
| 8 | 2,508 | 3,783 | |
| 9 | 2,401 | 7,516 | |
| 10 | 2,060 | 2,084 | |
| 11 | 1,876 | 3,936 | |
| 12 | 1,773 | 2,314 | |
| 13 | 1,672 | 6,236 | |
| 14 | 1,624 | 2,407 | |
| 15 | 1,553 | 1,554 | |
| 16 | 1,323 | 2,673 | |
| 17 | 1,223 | 2,658 | |
| 18 | 1,113 | 1,323 | |
| 19 | 1,081 | 3,864 | |
| 20 | 935 | 1,906 | |
Africa
There are important mangrove swamps in Kenya, Tanzania, the Democratic Republic of the Congo (DRC), and Madagascar, with the latter even admixing at the coastal verge with dry deciduous forests.
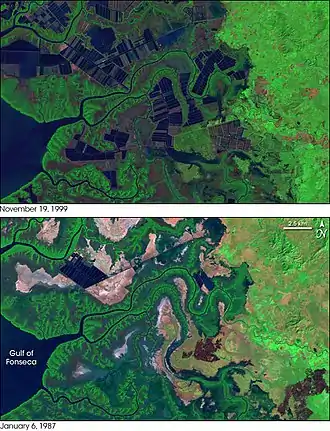
Nigeria has Africa's largest mangrove concentration, spanning 36,000 km2. Oil spills and leaks have destroyed many in the last 50 years, damaging the local fishing economy and water quality.[29]
Along the coast of the Red Sea, both on the Egyptian side and in the Gulf of Aqaba, mangroves composed primarily of Avicennia marina and Rhizophora mucronata[30] grow in about 28 stands that cover about 525 hectares. Almost all Egyptian mangrove stands are now protected.
There are mangroves off the east coast of South Africa extending as far south as the Tylomnqa River (33°13'26.1"S 27°34'50.2"E). Some mangrove stands exist in the St Lucia estuary within iSimangaliso Wetland Park.
Americas
Mangroves live in many parts of the tropical and subtropical coastal zones of North and South America.
Central America and the Caribbean
Mangroves occur on the Pacific and Caribbean coasts of Belize, Costa Rica, El Salvador, Guatemala, Honduras, Nicaragua, and Panama. Mangroves can also be found in many of the Antilles including Puerto Rico,[31] Cuba, and Hispaniola,[32] as well as other islands in the West Indies such as the Bahamas.
Belize
The nation of Belize has the highest overall percentage of forest cover of any of the Central American countries.[33] In terms of Belize's mangrove cover—which assumes the form not only of mangrove 'forest', but also of scrubs and savannas, among others[34]—a 2010 satellite-based study of Belize's mangroves by the World Wildlife Fund (WWF) and the Water Center for the Humid Tropics of Latin America and the Caribbean found, in 2010, mangroves covered some 184,548 acres (74,684 hectares) or 3.4% of Belize's territory.[35]
In 1980, by contrast, mangrove cover stood at 188,417 acres (76,250 hectares)—also 3.4% of Belize's territory, although based on the work of mangrove researcher Simon Zisman,[36] Belize's mangrove cover in 1980 was estimated to represent 98.7% of the precolonial extent of those ecosystems. Belize's mangrove cover in 2010 was thus estimated to represent 96.7% of the precolonial cover.[35] Assessing changes in Belize's mangrove cover over a 30-year period was possible because of Belize's participation in the Regional Visualization and Monitoring System, a regional observatory jointly implemented by CATHALAC, RCMRD, ICIMOD, NASA, USAID, and other partners.[37]
Continental United States
Mangrove communities, including red (Rhizophora mangle) and white (Laguncularia racemosa) mangroves, are confined in the continental United States to the Florida peninsula, up to 29 degrees North latitude (see Florida mangroves). Black mangroves (Avicennia germinans), which are more cold-tolerant than other mangrove species, grow as far north as 30 degrees North latitude on the East coast of Florida, and at isolated areas along the wider Gulf Coast, including Texas.[38]
The trimming of mangroves in Florida is regulated, and altering a mangrove without a permit (removing or cutting the plant so much that it dies or is defoliated) is prohibited and carries a fine.[39]
Mexico
In Mexico, four species of mangrove predominate: Rhizophora mangle, Laguncularia racemosa, Avicennia germinans and Conocarpus erectus.[40] During an inventory conducted by CONABIO between 2006 and 2008, 770,057 hectares of mangrove were counted.[41] Of this total, 55% are located in the Yucatán Peninsula.[41]
Significant mangals include the Marismas Nacionales-San Blas mangroves found in Sinaloa and Nayarit.
South America
Brazil contains approximately 26,000 km2 of mangals, 15% of the world's total.
Ecuador has substantial remaining mangrove forests in the provinces of El Oro, Guayas, Manabi and Esmeraldas with limited forest remaining in Santa Elena.[42] The northern portion of Esmeraldas province has a large pristine mangrove forest that is preserved as the Reserva Ecológica Cayapas-Mataje (REMACAN) and is an original Ramsar site.[43] This forest is the most preserved within Ecuador and likely the most pristine forest along the Pacific Coast of the Americas.[44]
The only other major mangrove holding in Esmeraldas is in-and-around the community of Muisne and the Rio Muisne Estuary Swampland Wildlife Refuges.[46] The mangroves in-and-around the estuaries of Muisne have decreased in area from 3222 ha in 1971 to 1065 ha as of 2005, during this time commercial shrimp aquaculture has become the dominant land-cover within this estuary environment.[47]
On the border of Esmeraldas province and Manabí province is a formerly large area of mangrove within Cojimies Estuary. The mangroves in this estuary are some of the most degraded in Ecuador with only 19% of 1971 mangrove area remaining as of 1998, although mangrove has recovered since this date.[44] Within Manabí the major mangrove holding estuary is the Chone estuary situated near the city of Bahía de Caráquez. Again, Chone has undergone substantial mangrove deforestation since the advent of commercial aquaculture in Ecuador.[47] Although mangrove loss appears to have halted in this estuary and mangrove regrowth driven by local fisherman is now occurring.[48]
Peru has 2 small regions of mangrove located in the Department of Tumbes, north-west of the country on the Ecuadorian Border, and also in the Piura region, where the "Manglares de Vice" in the Sechura Province of Piura is the southernmost region of the Pacific to hold mangroves.[1]
Venezuela's northern Caribbean island, Margarita, possesses mangrove forests in the Parque nacional Laguna de La Restinga. Venezuela has 4% of the world's mangroves, with an extension of 6735 km2.[49]
Colombia possesses large mangrove forests on both its Caribbean and Pacific coasts.
The Mangrove forests of Suriname have a height of 20–25 m and are found mainly in the coastal area. There are six types of mangroves, namely two types of parwa or black mangroves, three types of red mangroves (mangro) and a small mangrove species (white mangrove, akira or tjila).[50]
Indo-Malaya Ecozone
Mangroves occur on Asia's south coast, throughout the Indian subcontinent, in all Southeast Asian countries, and on islands in the Indian Ocean, Persian Gulf, Arabian Sea, Bay of Bengal, South China Sea, East China Sea and the Pacific. The mangal is particularly prevalent in the deltas of large Asian rivers. The Sundarbans is the largest mangrove forest in the world, located in the Ganges River delta in Bangladesh and West Bengal, India.
The Pichavaram mangroves in Tamil Nadu is India's one of the largest mangrove forests. The Bhitarkanika Mangroves Forest of Odisha, by the Bay of Bengal, is India's second largest mangrove forest. Other major mangals occur on the Andaman and Nicobar Islands and the Gulf of Kutch in Gujarat.[51]
Mangroves occur in certain muddy swampy islands of Maldives.[52]
On the Malay Peninsula, mangroves cover an estimated 1,089.7 square kilometres (420.7 sq mi), while most of the remaining 5,320 square kilometres (2,054 sq mi) mangroves in Malaysia are on the island of Borneo.[53]
In Vietnam, mangrove forests grow along the southern coast, including two forests: the Cần Giờ Mangrove Forest biosphere reserve and the U Minh mangrove forest in the sea and coastal region of Kiên Giang, Cà Mau and Bạc Liêu provinces. The mangrove forests of the Bay of Kompong Som (maki) in Cambodia are of major ecological and cultural importance, as the human population relies heavily on the crabs and fish that live in the roots.[54]
The three most important mangrove forests of Taiwan are: Tamsui River in Taipei, Jhonggang River in Miaoli and the Sihcao Wetlands in Tainan. According to research, four main types of mangrove exist in Taiwan.[55] Some places have been developed as scenic areas, such as the log raft routes in Sihcao.
The most extensive mangrove forests of the Ryukyu Islands in East China Sea occur on Iriomote Island of the Yaeyama Islands, Okinawa, Japan.[56] Seven types of mangroves are recognised on Iriomote Island.[57]
The northern limit of mangrove forests in the Indo-Malaya Ecozone is considered to be Tanegashima Island, Kyushu, Japan.[58]
Indonesia
In the Indonesian Archipelago, mangroves occur around much of Papua province, Sumatra, Borneo, Sulawesi, Maluku and the surrounding islands. Further north, they are found along the coast of the Malay Peninsula. Indonesia has around 9.36 million hectares of mangrove forests, but 48% is categorized as 'moderately damaged' and 23% as 'badly damaged'.[59]
Arabian Peninsula
The wide and shallow shelf on Yemen's Red Sea coast consists of unconsolidated sediments, which support 11 species of seagrasses up to 500 m (1,600 ft) offshore on the shoreward side of sand spits, islands and reefs. Fragmented mangrove patches cover around 3,000–5,000 ha (7,400–12,400 acres) along the coast, are 100–200 m (330–660 ft) wide along a 122 km (76 mi) stretch. The mangrove forest of Kamaran island was logged in the early 20th century, and the timber used for construction in Aden.[60]
The mangrove forests that cover thousands of hectares of land along the shoreline of the United Arab Emirates are an integral part of its coastal ecosystem. The Environment Agency – Abu Dhabi (EAD) is currently working on rehabilitation, conservation and protection of mangrove forests in seven key sites in Abu Dhabi including: Saadiyat Island, Jubail Island, Marawah Marine Biosphere Reserve (which also comprises Bu Tinah Island), Bu Sayeef Protected Area, Ras Gharab, the Eastern Corniche[61] and Ras Ghanada.
Oman supports large areas of mangroves near Muscat, in particular at Shinas, Qurm Park and Mahout Island. In Arabic, mangrove trees are known as qurm (قُرْم), thus the mangrove area in Oman is known as Qurm Park. A small mangrove area is present in the Kingdom of Bahrain.
Iran
Mangrove forests are present in Iran between 25°11′N to 27°52′N, in the northern parts of the Persian Gulf and Sea of Oman. Pockets of the biome extend (from southwest to southeast) along the shores of the maritime provinces Bushehr, Hormozgan, and Sistan and Balouchestan. Forests on and near the island of Qeshm in the Persian Gulf are dominated by the species Avicennia marina, known locally as the "hara" or "harra" tree, and cover an area of approximately 20 by 20 km (12 by 12 mi). This area is protected as the UNESCO Hara Biosphere Reserve, where commercial use is restricted to fishing (mainly shrimp), tourist boat trips, and limited mangrove cutting for animal feed.
India
As per the ISFR 2017 report, the total area of mangrove cover of India is 4921 km2, (181 km2 positively changed with respect to 2015 mangrove cover assessment) which contributes 3.3% to the global mangrove cover.[62] The deltas of the Ganges, Mahanadi, Krishna, Godavari, and Kaveri rivers contain mangrove forests. Backwaters in Kerala have high density of mangrove forest on the shores.[63] Indian mangroves consist of 46 species (4 of which are natural hybrids) belonging to 22 genera and 14 families, representing about 57% of the world's mangrove species.[64]
The following table shows the prevalence of mangroves in the states of India and the total area covered by them in square kilometres.[65]
| Rank | States/UTs with highest mangrove cover in 2017 | Total mangrove cover in km2 in 2015 | 2017[66][62] |
|---|---|---|---|
| 1 | West Bengal | 2,106 | 2114 |
| 2 | Gujarat | 1,107 | 1140 |
| 3 | Andaman And Nicobar Islands | 617 | 617 |
| 4 | Andhra Pradesh | 367 | 404 |
| 5 | Maharashtra | 222 | 304 |
| 6 | Odisha | 231 | 243 |
| 7 | Tamil Nadu | 47 | 49 |
| 8 | Goa | 26 | 26 |
| 9 | Kerala | 9 | 9 |
| 10 | Karnataka | 3 | 10 |
Baratang Island
The Baratang Island mangroves are located within the Andaman and Nicobar Islands. The mangrove swamps of Baratang Island are situated between Middle and South Andaman Island.[67]
Bhitarkanika
The Bhitarkanika mangroves form India's second largest forest, located in the state of Odisha. Bhitarkanika is created by the two river deltas of Brahmani and Baitarani river and one of the important Ramsar Wetland in India. It is also the home of saltwater crocodiles and nesting olive ridley sea turtles.[68][67]
Godavari-Krishna
The Godavari-Krishna mangroves lie in the delta of the Godavari and Krishna rivers in the state of Andhra Pradesh. Mangroves ecoregion is under protection for Calimere Wildlife and Pulicat Lake Bird Sanctuary.[67]
Mumbai
The megacity Mumbai has mangroves on its coastline along the west coast of India. A total of 10 mangrove species were reported in this area, dominated by Avicennia marina.[69] These mangroves support a rich diversity of life forms, especially molluscs.[70] The total mangrove area in Mumbai is 50 km2.[66] Mumbai's single largest mangrove belt is the western bank of Thane Creek. The Government of Maharashtra has declared much of the area on the western bank of Thane Creek as the Thane Creek Flamingo Sanctuary.[71] Mangrove areas on the government lands are governed by the Maharashtra Forest Department. An extensive area of mangroves on the private lands in Vikhroli has been conserved by Soonabai Pirojsha Godrej Marine Ecology Centre, Vikhroli, Mumbai.[72]
Pichavaram

The Pichavaram mangroves are situated at Pichavaram near Chidambaram in the state of Tamil Nadu. Pichavaram ranks amongst one of the most exquisite scenic spots in Tamil Nadu and has many species of aquatic birds.[67]
Sundarbans
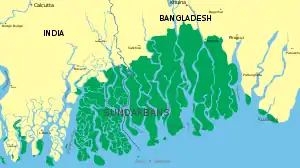

The Sundarbans in the Ganges-Brahmaputra delta extend from the Hooghly River in West Bengal to the Baleswar River in Bangladesh, covering an area of about 10,000 km2 (3,900 sq mi). This area comprises closed and open mangrove forests, agriculturally used land, mudflats and barren land. It is intersected by tidal streams and channels. Four protected areas in the Sundarbans, viz Sundarbans National Park, Sundarbans West, Sundarbans South and Sundarbans East Wildlife Sanctuaries are enlisted as UNESCO World Heritage Sites. Biodiversity includes at least 27 mangrove species, 40 mammal, 35 reptile and 260 bird species. More than 2.5 million people are estimated to live in the vicinity of the Sundarbans, making them one of the world's most densely populated areas.[73]
It is the largest mangrove region and the largest single block of tidal halophytic mangrove forest in the world. Sundri (Heritiera fomes) trees provide durable hard timber. Palm, coconut, keora, agar, also grow in some parts of the delta. India's mangrove forests are habitat for saltwater crocodile (Crocodylus porosus), turtles, and snakes. This region is part of the Great Sundarbans and covers a National Park, Tiger reserve and a Biosphere Reserve.[68] Sundarbans was designated a Ramsar site on May 21, 1992. The fertile soils of the delta have been subject to intensive human use for centuries, and the ecoregion has been mostly converted to intensive agriculture, with few enclaves of forest remaining. The remaining forests, together with the Sundarbans mangroves, are important habitat for the endangered tiger. Additionally, the Sundarbans serves a crucial function as a protective flood barrier for the millions of inhabitants in and around Kolkata against the result of cyclone activity. Sundarbans is home to many different species of birds, mammals, insects, reptiles and fish. It is estimated that there may be found more than 120 species of fish and over 260 species of birds and more than fifty species of reptiles and eight amphibians.
Pakistan


Pakistani mangroves are located mainly along the delta of the Indus River (the Indus River Delta-Arabian Sea mangroves ecoregion). Major mangrove forests are found on the coastline of the provinces of Sindh and Balochistan.
In Karachi, land reclamation projects have led to the cutting down of mangrove forests for commercial and urban development. On 22 June 2013, Sindh Forest Department, Govt. of Sindh, Pakistan, with the help of 300 local coastal volunteer planters set the Guinness World Record by planting 847,250 mangrove saplings at Kharo Chan, Thatta, Sindh, Pakistan in a little over 12 hours. This is the highest number of saplings planted within a day under the Guinness World Record category of "Maximum Number of Trees Planted in a Day".
Sindh Forest Department, Government of Sindh Mangrove has played pioneer role for the conservation and protection of the mangroves in the Indus Delta since late 1950s when it was handed over the areas. A breakthrough success is the re-introduction of Rhizophora mucronata into the Indus Delta, which had become extinct there. More recently, a threatened mangrove shrub, Ceriops tagal, has also been successfully re-introduced. A third species, Aegiceras corniculatum, is under trials at the nursery stage.
A major initiative is currently underway in Sindh, Pakistan, to rehabilitate degraded mangrove mudflats. Since 2010 alone, around 55,000 hectares of former mangrove forest have been planted and rehabilitated. During this period, through concerted efforts and a rigorous conservation policy adopted by the Sindh Forest Department, the government of Sindh and the federal government, a mangrove resource base of 150,000 plus hectares has been created, with the support of local coastal communities. International organizations like IUCN and WWF are also playing critical role to support this initiative. Other achievements include: (1) Declaring all the mangrove forests in the Indus Delta as Protected Forests in December 2010; Constitution of a Mangrove Conservation Committee at the provincial level which includes all stakeholders as members and overall awareness of the importance of mangroves and its ecosystem.[74]
Australia and New Guinea
Australia and Papua New Guinea both rank in the top five mangrove holding nations globally.[75] More than five species of Rhizophoraceae grow in Australasia,[76] with particularly high biodiversity on the island of New Guinea and northern Australia.[76]
As of 2012 Australia has slightly below 1 million ha of mangrove [75] and Papua New Guinea has just under approximately 500,000 ha +- 12% (CI 0.9, n = 7) of mangrove.[75]
New Zealand
New Zealand also has mangrove forests extending to around 38°S (similar to Australia's latitudinal limit): the southernmost examples are at Raglan Harbour (37°48′S) on the west coast and Ohiwa Harbour (near Ōpōtiki, 38°00′S) on the east coast. Avicennia marina australasica is the only mangrove in New Zealand.
Pacific Islands
Twenty-five species of mangrove are found on various Pacific islands, with extensive mangals on some islands. Mangals on Guam, Palau, Kosrae and Yap have been badly affected by development.[77]
Mangroves are not native to Hawaii, but the red mangrove, Rhizophora mangle, and Oriental mangrove, Bruguiera sexangula, have been introduced and are now naturalized.[78] Both species are considered invasive species and classified as pests by the University of Hawaii Botany Department.[79]
Exploitation and conservation


Adequate data are only available for about half of the global area of mangroves. However, of those areas for which data has been collected, it appears that 35% of the mangroves have been destroyed.[80] Assessments of global variation in mangrove loss indicates that national regulatory quality mediates how different drivers and pressures influence loss rates.[81] The United Nations Environment Programme and Hamilton (2013), estimate that shrimp farming causes approximately a quarter of the destruction of mangrove forests.[82][83] Likewise, the 2010 update of the World Mangrove Atlas indicated that approximately one fifth of the world's mangrove ecosystems have been lost since 1980,[84] although this rapid loss rate appears to have decreased since 2000 with global losses estimated at between 0.16% and 0.39% annually between 2000 and 2012.[28] Despite global loss rates decreasing since 2000, Southeast Asia remains an area of concern with loss rates between 3.58% and 8.08% between 2000 and 2012.[28]
Grassroots efforts to save mangroves from development are becoming more popular as their benefits become more widely known. In the Bahamas, for example, active efforts to save mangroves are occurring on the islands of Bimini and Great Guana Cay. In Trinidad and Tobago as well, efforts are underway to protect a mangrove threatened by the construction of a steelmill and a port. In Thailand, community management has been effective in restoring damaged mangroves.[85] Within northern Ecuador mangrove regrowth is reported in almost all estuaries and stems primarily from local actors responding to earlier periods of deforestation in the Esmeraldas region.[48]
Mangroves have been reported to be able to help buffer against tsunami, cyclones, and other storms, and as such may be considered a flagship system for ecosystem-based adaptation to the impacts of climate change. One village in Tamil Nadu was protected from tsunami destruction—the villagers in Naluvedapathy planted 80,244 saplings to get into the Guinness Book of World Records. This created a kilometre-wide belt of trees of various varieties. When the 2004 tsunami struck, much of the land around the village was flooded, but the village itself suffered minimal damage.[86]
Reforestation

In some areas, mangrove reforestation and mangrove restoration is also underway. Red mangroves are the most common choice for cultivation, used particularly in marine aquariums in a sump to reduce nitrates and other nutrients in the water. Mangroves also appear in home aquariums, and as ornamental plants, such as in Japan.
In Senegal, Haïdar El Ali has started the fr project, which (amongst others) focuses on reforesting several areas with mangroves.[87]
The Manzanar Mangrove Initiative is an ongoing experiment in Arkiko, Eritrea, part of the Manzanar Project founded by Gordon H. Sato, establishing new mangrove plantations on the coastal mudflats. Initial plantings failed, but observation of the areas where mangroves did survive by themselves led to the conclusion that nutrients in water flow from inland were important to the health of the mangroves. Trials with the Eritrean Ministry of Fisheries followed, and a planting system was designed to provide the nitrogen, phosphorus, and iron missing from seawater.[88][89]
The propagules are planted inside a reused galvanized steel can with the bottom knocked out; a small piece of iron and a pierced plastic bag with fertilizer containing nitrogen and phosphorus are buried with the propagule. As of 2007, after six years of planting, 700,000 mangroves are growing; providing stock feed for sheep and habitat for oysters, crabs, other bivalves, and fish.[88][89]
Seventy percent of mangrove forests have been lost in Java, Indonesia. Mangroves formerly protected the island's coastal land from flooding and erosion.[90] Wetlands International, an NGC based in the Netherlands, in collaboration with nine villages in Demak where lands and homes had been flooded, began reviving mangrove forests in Java. Wetlands International introduced the idea of developing tropical versions of techniques traditionally used by the Dutch to catch sediment in North Sea coastal salt marshes.[90] Originally, the villagers constructed a sea barrier by hammering two rows of vertical bamboo poles into the seabed and filling the gaps with brushwood held in place with netting. Later the bamboo was replaced by PVC pipes filled with concrete. As sediment gets deposited around the brushwood, it serves to catch floating mangrove seeds and provide them with a stable base to germinate, take root and regrow. This creates a green belt of protection around the islands. As the mangroves mature, more sediment is held in the catchment area; the process is repeated until a mangrove forest has been restored. Eventually the protective structures will not be needed.[90] By late 2018, 10 miles (16 km) of brushwood barriers along the coastline had been completed.[90]
A concern over reforestation is that although it supports increases in mangrove area it may actually result in a decrease in global mangrove functionality and poor restoration processes may result in longer term depletion of the mangrove resource.[91]
National studies
In terms of local and national studies of mangrove loss, the case of Belize's mangroves is illustrative in its contrast to the global picture. A recent, satellite-based study[35]—funded by the World Wildlife Fund and conducted by the Water Center for the Humid Tropics of Latin America and the Caribbean (CATHALAC)—indicates Belize's mangrove cover declined by a mere 2% over a 30-year period. The study was born out of the need to verify the popular conception that mangrove clearing in Belize was rampant.[92]
Instead, the assessment showed, between 1980 and 2010, under 4,000 acres (16 km2) of mangroves had been cleared, although clearing of mangroves near Belize's main coastal settlements (e.g. Belize City and San Pedro) was relatively high. The rate of loss of Belize's mangroves—at 0.07% per year between 1980 and 2010—was much lower than Belize's overall rate of forest clearing (0.6% per year in the same period).[93] These findings can also be interpreted to indicate Belize's mangrove regulations (under the nation's)[94] have largely been effective. Nevertheless, the need to protect Belize's mangroves is imperative, as a 2009 study by the World Resources Institute (WRI) indicates the ecosystems contribute US$174–249 million per year to Belize's national economy.[95]
International research
In May 2019, ORNL DAAC News announced that NASA's Carbon Monitoring System (CMS), using new satellite-based maps of global mangrove forests across 116 countries, had created a new dataset to characterize the "distribution, biomass, and canopy height of mangrove-forested wetlands".[96][97] Mangrove forests move carbon dioxide "from the atmosphere into long-term storage" in greater quantities than other forests, making them "among the planet's best carbon scrubbers" according to a NASA-led study.[97][98]
See also
References
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J. & Duke, N. (2010). "Status and distribution of mangrove forests of the world using earth observation satellite data" (PDF). Global Ecology and Biogeography. 20 (1): 154–159. doi:10.1111/j.1466-8238.2010.00584.x. Retrieved 2012-02-08.
- Flowers, T. J.; Colmer, T. D. (2015). "Plant salt tolerance: adaptations in halophytes" (PDF). Annals of Botany. 115 (3): 327–331. doi:10.1093/aob/mcu267. PMC 4332615. PMID 25844430.
- Hogarth, Peter J. (1999) The Biology of Mangroves Oxford University Press, Oxford, England, ISBN 0-19-850222-2.
- {{cite web|url=http://www.nhmi.org/mangroves/phy.htm |title=Morphological and Physiological Adaptations: Florida mangrove website |publisher=Nhmi.org |access-date=2012-02-08 |url-status=dead |archive-url=https://web.archive.org/web/20120204222702/http://www.nhmi.org/mangroves/phy.htm |archive-date=2012-02-04 }Mangrove forests move carbon dioxide "from the atmosphere into long-term storage" in greater quantities than other forests, making them "among the planet's best carbon scrubbers" according to a NASA-led study based on satellite data.
- Rafinesque, C.S. (1836). The American Nations. 1. C. S. Rafinesque. p. 244.
- "Mangal (Mangrove). World Vegetation. Mildred E. Mathias Botanical Garden, University of California at Los Angeles". Botgard.ucla.edu. Archived from the original on 2012-02-09. Retrieved 2012-02-08.
- Sievers, Michael; Brown, Christopher J.; Tulloch, Vivitskaia J.D.; Pearson, Ryan M.; Haig, Jodie A.; Turschwell, Mischa P.; Connolly, Rod M. (2019). "The Role of Vegetated Coastal Wetlands for Marine Megafauna Conservation". Trends in Ecology & Evolution. 0 (9): 807–817. doi:10.1016/j.tree.2019.04.004. hdl:10072/391960. ISSN 0169-5347. PMID 31126633.
- Mazda, Y.; Kobashi, D.; Okada, S. (2005). "Tidal-Scale Hydrodynamics within Mangrove Swamps". Wetlands Ecology and Management. 13 (6): 647–655. CiteSeerX 10.1.1.522.5345. doi:10.1007/s11273-005-0613-4. S2CID 35322400.
- Danielsen, F.; Sørensen, M. K.; Olwig, M. F; Selvam, V; Parish, F; Burgess, N. D; Hiraishi, T.; Karunagaran, V. M.; Rasmussen, M. S.; Hansen, L. B.; Quarto, A.; Suryadiputra, N. (2005). "The Asian Tsunami: A Protective Role for Coastal Vegetation". Science. 310 (5748): 643. doi:10.1126/science.1118387. PMID 16254180. S2CID 31945341.
- Takagi, H.; Mikami, T.; Fujii, D.; Esteban, M.; Kurobe, S. (2016). "Mangrove forest against dyke-break-induced tsunami on rapidly subsiding coasts". Natural Hazards and Earth System Sciences. 16 (7): 1629–1638. Bibcode:2016NHESS..16.1629T. doi:10.5194/nhess-16-1629-2016.
- Massel, S. R.; Furukawa, K.; Brinkman, R. M. (1999). "Surface wave propagation in mangrove forests". Fluid Dynamics Research. 24 (4): 219. Bibcode:1999FlDyR..24..219M. doi:10.1016/s0169-5983(98)00024-0.
- Mazda, Y.; Wolanski, E.; King, B.; Sase, A.; Ohtsuka, D.; Magi, M. (1997). "Drag force due to vegetation in mangrove swamps". Mangroves and Salt Marshes. 1 (3): 193. doi:10.1023/A:1009949411068. S2CID 126945589.
- Baird, A. (2006). "False Hopes and Natural Disasters". The New York Times.
- Dahdouh-Guebas, F.; Jayatissa, L. P.; Di Nitto, D.; Bosire, J. O.; Lo Seen, D.; Koedam, N. (2005). "How effective were mangroves as a defence against the recent tsunami?". Current Biology. 15 (12): R443–447. doi:10.1016/j.cub.2005.06.008. PMID 15964259. S2CID 8772526.
- Bos, A. R.; Gumanao, G. S.; Van Katwijk, M. M.; Mueller, B.; Saceda, M. M.; Tejada, R. L. P. (2010). "Ontogenetic habitat shift, population growth, and burrowing behavior of the Indo-Pacific beach star, Archaster typicus (Echinodermata; Asteroidea)". Marine Biology. 158 (3): 639–648. doi:10.1007/s00227-010-1588-0. PMC 3873073. PMID 24391259.
- Encarta Encyclopedia 2005. "Seashore", by Heidi Nepf.
- Skov, M. W.; Hartnoll, R. G. (2002). "Paradoxical selective feeding on a low-nutrient diet: Why do mangrove crabs eat leaves?". Oecologia. 131 (1): 1–7. Bibcode:2002Oecol.131....1S. doi:10.1007/s00442-001-0847-7. PMID 28547499. S2CID 23407273.
- Vane, C. H.; Kim, A. W.; Moss-Hayes, V.; Snape, C. E.; Diaz, M. C.; Khan, N. S.; Engelhart, S. E.; Horton, B. P. (2013). "Degradation of mangrove tissues by arboreal termites (Nasutitermes acajutlae) and their role in the mangrove C cycle (Puerto Rico): Chemical characterization and organic matter provenance using bulk δ13C, C/N, alkaline CuO oxidation-GC/MS, and solid-state" (PDF). Geochemistry, Geophysics, Geosystems. 14 (8): 3176. Bibcode:2013GGG....14.3176V. doi:10.1002/ggge.20194.
- Versteegh, G. J. M.; et al. (2004). "Taraxerol and Rhizophora pollen as proxies for tracking past mangrove ecosystems". Geochimica et Cosmochimica Acta. 68 (3): 411–422. Bibcode:2004GeCoA..68..411V. doi:10.1016/S0016-7037(03)00456-3.
- Hamilton, S. E.; Friess, D. A. (2018). "Global carbon stocks and potential emissions due to mangrove deforestation from 2000 to 2012". Nature Climate Change. 8 (3): 240–244. arXiv:1611.00307. Bibcode:2018NatCC...8..240H. doi:10.1038/s41558-018-0090-4. S2CID 89785740.
- Hochard, Jacob P.; Hamilton, Stuart; Barbier, Edward B. (2019-06-03). "Mangroves shelter coastal economic activity from cyclones". Proceedings of the National Academy of Sciences. 116 (25): 12232–12237. doi:10.1073/pnas.1820067116. ISSN 0027-8424. PMC 6589649. PMID 31160457.
- "Distribution of coral, mangrove and seagrass diversity". Maps.grida.no. Archived from the original on 2010-03-05. Retrieved 2012-02-08.
- Gray, L. Joseph; et al. (2010). "Sacrificial leaf hypothesis of mangroves" (PDF). ISME/GLOMIS Electronic Journal. GLOMIS. Retrieved 21 January 2012.
- "Calfo, Anthony (2006). Mangroves for the Marine Aquarium". Reefkeeping.com. Retrieved 2012-02-08.
- Ricklefs, R. E.; A. Schwarzbach; S. S. Renner (2006). "Rate of lineage origin explains the diversity anomaly in the world's mangrove vegetation" (PDF). American Naturalist. 168 (6): 805–810. doi:10.1086/508711. PMID 17109322. Archived from the original (PDF) on 2013-06-16.
- Hamilton, Stuart E.; Castellanos-Galindo, Gustavo A.; Millones-Mayer, Marco; Chen, Mara (2018), Makowski, Christopher; Finkl, Charles W. (eds.), "Remote Sensing of Mangrove Forests: Current Techniques and Existing Databases", Threats to Mangrove Forests, Springer International Publishing, 25, pp. 497–520, doi:10.1007/978-3-319-73016-5_22, ISBN 9783319730158
- "Ao Phang-nga National Park". National Park website. Archived from the original on 20 October 2014. Retrieved 24 November 2014.
- Hamilton, Stuart E; Casey, Daniel (2016). "Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21)". Global Ecology and Biogeography. 25 (6): 729–38. arXiv:1412.0722. doi:10.1111/geb.12449. S2CID 55999275.
- O'Neill, Tom (February 2007). "Curse of the Black Gold: Hope and betrayal in the Niger Delta". National Geographic. 211 (2): 88–117. Archived from the original on 5 December 2010.
- Ali A. Gab-Alla; Ishrak, K. Khafagi; Waleed, M. Morsy; Moustafa M. Fouda (2010). "Ecology of Avicennia marina mangals along Gulf of Aqaba, South Sinai, Red Sea" (PDF). Egypt J. Aquat. Biol. & Fish. 14 (2): 79–93. doi:10.21608/ejabf.2010.2063. Archived from the original (PDF) on 2014-02-20. Retrieved 25 January 2013.
- Departamento de Recursos Naturales y Ambientales. "Los Maglares – Hojas de Nuestro Ambiente" (PDF). www.drna.gobierno.pr. Departamento de Recursos Naturales y Ambientales. Archived from the original (PDF) on 9 June 2015. Retrieved 8 June 2015.
- Meyer-Arendt, Klaus; Byrd. S; Hamilton, SE (October 2013). "Mangrove deforestation in the Dominican Republic, 1969 to 2012" (PDF). GLOMIS / ISME Electronic Journal. 1 (1): 1. Retrieved 1 November 2013.
- "Vreugdenhil, D., Meerman, J., Meyrat, A., Gómez, L. D., and D. J. Graham "Map of the Ecosystems of Central America: Final Report" World Bank, Washington, DC. 56 pp". 2002. Retrieved 2014-01-25.
- Murray, M.R; Zisman, S.A; Furley, P.A; Munro, D.M; Gibson, J; Ratter, J; Bridgewater, S; Minty, C.D; Place, C.J (2003). "The mangroves of Belize". Forest Ecology and Management. 174 (1–3): 265–279. doi:10.1016/s0378-1127(02)00036-1.
- Cherrington EA, Hernandez BE, Trejos NA, Smith OA, Anderson ER, Flores AI, Garcia BC (2010). Identification of Threatened and Resilient Mangroves in the Belize Barrier Reef System (PDF). Water Center for the Humid Tropics of Latin America and the Caribbean (CATHALAC) / Regional Visualization & Monitoring System (SERVIR) (Report). Technical report to the World Wildlife Fund. Archived from the original (PDF) on July 31, 2013.
- Zisman, S.A. 1998. "Sustainability or Status Quo: Elite Influence and the Political Ecology of Mangrove Exploitation in Belize." Doctoral dissertation, Department of Geography, University of Edinburgh. Edinburgh, Scotland.
- "NASA – NASA, USAID Expand Web-Based Environmental Monitoring System". Nasa.gov. 2010-10-05. Retrieved 2012-02-08.
- "Mangroves" (PDF). South Florida Ecological Services Field Office. U.S. Fish & Wildlife Service. pp. 3–520, 3–522. Retrieved January 27, 2021.
- Fisher, Kellyalexis (1998). "Man Let 'em Grow: The State of Florida Mangrove Laws". Florida Bar Journal.
- "Mangroves of Mexico". Biodiversidad.gob.mx. Archived from the original on 17 April 2016. Retrieved 10 February 2016.
- "Monitoring program". Biodiversidad.gob.mx. Archived from the original on 15 February 2016. Retrieved 10 February 2016.
- Hamilton, Stuart (2011). The impact of shrimp farming on mangrove ecosystems and local livelihoods along the Pacific coast of Ecuador. p. 194. ISBN 978-1249871736.
- "Ramsar sites Database". The Ramsar convention on wetlands. Archived from the original on 2013-04-16.
- Hamilton, Stuart; Clare Stankwitz (2012). "Examining the relationship between international aid and mangrove deforestation in coastal Ecuador from 1970 to 2006". Land Use Science. 7 (2): 177–202. doi:10.1080/1747423x.2010.550694. S2CID 140159850.
- "O Valor da Opção de Preservação do Parque dos Manguezais em Recife-PE: Uma Utilização do Método de Opções Reais" (PDF) (in Portuguese). ANPEC. Retrieved 2015-06-04.
- "Ecuador:Mangrove Restoration in Muisne". Global Restoration Network. Archived from the original on 2013-05-11. Retrieved 20 December 2012.
- Hamilton, Stuart (2011-01-01). "Quantifying mangrove deforestation in Ecuador's northern estuaries since the advent of commercial aquaculture". Glomis / ISME. 9 (1): 1–3. Retrieved 20 December 2012.
- Hamilton, S. & S. Collins (2013) Las respuestas a los medios de subsistencia deforestación de los manglares en las provincias del norte de Ecuador. Bosque 34:2
- "Mangroves of Venezuela". azulambientalistas.org. Retrieved 2012-12-13.
- Environmental statistics of Suriname 2014. http://www.statistics-suriname.org/index.php/statistieken/downloads/category/34-milieu-publicatie-2012 Archived 2015-02-11 at the Wayback Machine
- Mangroves of India Archived 2006-11-18 at the Wayback Machine – URL retrieved November 26, 2006
- Xavier Romero-Frias, The Maldive Islanders, A Study of the Popular Culture of an Ancient Ocean Kingdom. Barcelona 1999, ISBN 84-7254-801-5.
- Augustin, Sean (15 September 2014). "Mangroves protect Malaysia's coast, but also shield illegals". The Rakyat Post. Kuala Lumpur, Malaysia. Archived from the original on 15 September 2014. Retrieved 15 September 2014.
- Mark Spalding, World Atlas of Mangroves (London: Routledge, 2010), 104 and 130-33. ISBN 1136530967
- Fan, K.C. "Mangroves in Taiwan: current status and restoration projects" (PDF).
- Nakasuga, Tsuneo (December 1979). "Analysis of the mangrove stand (Department of Forestry)". 琉球大学農学部学術報告. University of the Ryukyus. 26: 413–519. Retrieved 2015-08-19.
- "Iriomote Island and the Iriomote Wildcat". Japanese Ministry of Foreign Affairs (MOFA). Retrieved 2015-08-19.
- Somiya, Kazuo. "Conservation of landscape and culture in southwestern islands of Japan". Naha Nature Conservation Office, Ministry of the Environment. Archived from the original (PDF) on 2015-09-04. Retrieved 2015-08-19.
- "71% of Indonesian mangrove forests damaged: minister". The Jakarta Post. Archived from the original on 2012-01-18. Retrieved 2012-02-08.
- Rouphael, T.; Turak, E.; Brodie, J. (1998). "Seagrasses and Mangroves of Yemen's Red Sea" (PDF). In Dou Abal, A.; Rouphael, T. (eds.). Protection of Marine Ecosystems of the Red Sea Coast of Yemen. New York: UN publications. pp. 41–49.
- مرسى القرم الشرقي. Visit Abu Dhabi (in Arabic). Retrieved 2019-04-14.
- "Archived copy" (PDF). Archived from the original (PDF) on 2018-08-28. Retrieved 2018-11-02.CS1 maint: archived copy as title (link)
- "Chettuva in Thrissur: Flaunting Kerala's biggest mangrove forest". OnManorama. Retrieved 2018-08-28.
- http://tai2.ntu.edu.tw/taiwania/pdf/tai.2016.61.224.pdf%5B%5D%5B%5D
- "Top 10 Mangrove Forest in India".
- India State Forest Report, 2015
- "Top 5 Largest Mangrove And Swamp Forest in India".
- "Largest Wetland and Ramsar Sites in India".
- Kantharajan, G; Pandey, P.K; Krishnan, P; Ragavan, P; Jeevamani, J. Joyson Joe; Purvaja, R; Ramesh, R (2018). "Vegetative structure and species composition of mangroves along the Mumbai coast, Maharashtra, India". Regional Studies in Marine Science. 19: 1–8. doi:10.1016/j.rsma.2018.02.011.
- Kantharajan, G; Pandey, P.K; Krishnan, P; Deepak Samuel, V; Bharti, V.S; Purvaja, R (2017). "Molluscan diversity in the mangrove ecosystem of Mumbai, west coast of India". Regional Studies in Marine Science. 14: 102–11. doi:10.1016/j.rsma.2017.06.002.
- "Mumbai gets a flamingo sanctuary". The Hindu. 2015-08-08. Retrieved 2015-08-09.
- http://www.mangroves.godrej.com/%5B%5D%5B%5D
- Giri, C.; Pengra, B.; Zhu, Z.; Singh, A.; Tieszen, L. L. (2007). "Monitoring mangrove forest dynamics of the Sundarbans in Bangladesh and India using multi-temporal satellite data from 1973 to 2000". Estuarine, Coastal and Shelf Science. 73 (1–2): 91–100. Bibcode:2007ECSS...73...91G. doi:10.1016/j.ecss.2006.12.019.
- "By planting 750,000 mangroves, Pakistan claims new world record". Express Tribune. 22 June 2013. Archived from the original on 24 June 2013. Retrieved 23 June 2013.
- Hamilton, Stuart E.; Castellanos-Galindo, Gustavo A.; Millones-Mayer, Marco; Chen, Mara (2018). Threats to Mangrove Forests. Coastal Research Library. Springer, Cham. pp. 497–520. doi:10.1007/978-3-319-73016-5_22. ISBN 9783319730158.
- The world's mangroves, 1980–2005: a thematic study in the framework of the Global Forest Resources Assessment 2005 (FAO forestry paper #153(FAO) Rome. Food and Agriculture Organization of the United Nations (FAO). 2007. p. 37. ISBN 978-92-5-105856-5.
- Lloyd L. Loope. "Hawaii and the Pacific Islands". United States Geological Survey. Archived from the original on September 27, 2006.
- Allen, James A. & Krauss, Ken W. (2006). "Influence of propagule flotation longevity and light availability on establishment of introduced mangrove species in Hawai'i". Pacific Science. 60 (3): 367–376. doi:10.1353/psc.2006.0015. hdl:10125/22572. S2CID 53700898.
- Hawaiian Alien Plant Studies – URL retrieved November 28, 2006.
- Millennium Ecosystem Assessment (2005) Ecosystems and Human Well-being: Synthesis (p.2) Island Press, Washington, DC. World Resources Institute ISBN 1-59726-040-1
- Turschwell, Mischa P.; Tulloch, Vivitskaia J. D.; Sievers, Michael; Pearson, Ryan M.; Andradi-Brown, Dominic A.; Ahmadia, Gabby N.; Connolly, Rod M.; Bryan-Brown, Dale; Lopez-Marcano, Sebastian; Adame, Maria Fernanda; Brown, Christopher J. (2020-07-01). "Multi-scale estimation of the effects of pressures and drivers on mangrove forest loss globally". Biological Conservation. 247: 108637. doi:10.1016/j.biocon.2020.108637. ISSN 0006-3207.
- Botkin, D. and E. Keller (2003) Environmental Science: Earth as a living planet (p.2) John Wiley & Sons. ISBN 0-471-38914-5
- Hamilton, Stuart (2013). "Assessing the Role of Commercial Aquaculture in Displacing Mangrove Forest". Bulletin of Marine Science. 89 (2): 585–601. doi:10.5343/bms.2012.1069.
- "2010a. ""World Atlas of Mangroves" Highlights the Importance of and Threats to Mangroves: Mangroves among World's Most Valuable Ecosystems." Press release. Arlington, Virginia". The Nature Conservancy. Archived from the original on 2010-07-17. Retrieved 2014-01-25.
- "Thailand – Trang Province – Taking Back the Mangroves with Community Management | The EcoTipping Points Project". Ecotippingpoints.org. Retrieved 2012-02-08.
- "Tree News, Spring/Summer 2005, Publisher Felix Press". Treecouncil.org.uk. Retrieved 2012-02-08.
- "Oceanium de Dakar". Oceanium.blogspot.com. 2011-01-26. Retrieved 2012-02-08.
- Warne, Kennedy (February 2007). "Mangroves: Forests of the Tide". National Geographic. Tim Laman, photographer. National Geographic Society. Retrieved 2010-08-08.
- Sato, Gordon; Abraham Fisseha; Simon Gebrekiros; Hassan Abdul Karim; Samuel Negassi; Martin Fischer; Emanuel Yemane; Johannes Teclemariam & Robert Riley (2005). "A novel approach to growing mangroves on the coastal mud flats of Eritrea with the potential for relieving regional poverty and hunger". Wetlands. 25 (3): 776–779. doi:10.1672/0277-5212(2005)025[0776:ANATGM]2.0.CO;2.
- Pearce, Fred (May 2, 2019). "On Java's Coast, A Natural Approach to Holding Back the Waters". Yale E360. Retrieved 2019-05-15.
- Lee, Shing Yip; Hamilton, Stu; Barbier, Edward B.; Primavera, Jurgenne; Lewis, Roy R. (June 2019). "Better restoration policies are needed to conserve mangrove ecosystems". Nature Ecology & Evolution. 3 (6): 870–872. doi:10.1038/s41559-019-0861-y. ISSN 2397-334X. PMID 31036899. S2CID 139106235.
- "Pelican_Cays_Review" (PDF). Retrieved 2012-02-08.
- Cherrington, E.A., Ek, E., Cho, P., Howell, B.F., Hernandez, B.E., Anderson, E.R., Flores, A.I., Garcia, B.C., Sempris, E., and D.E. Irwin. 2010. "Forest Cover and Deforestation in Belize: 1980–2010." Water Center for the Humid Tropics of Latin America and the Caribbean. Panama City, Panama. 42 pp. Archived July 29, 2013, at the Wayback Machine
- Government of Belize (GOB). 2003. "Forests Act Subsidiary Laws." Chapter 213 in: Substantive Laws of Belize. Revised Edition 2003. Government Printer: Belmopan, Belize. 137 pp. Archived March 13, 2012, at the Wayback Machine
- Cooper, E.; Burke, L.; Bood, N. (2009). "Coastal Capital: Belize. The Economic Contribution of Belize's Coral Reefs and Mangroves" (PDF). Washington, DC: World Resources Institute. Retrieved 2014-06-23.
- "Mapping Global Mangrove Forests", ORNL DAAC News, May 6, 2019, retrieved May 15, 2019
- Rasmussen, Carol; Carlowicz, Mike (February 25, 2019), New Satellite-Based Maps of Mangrove Heights (Text.Article), retrieved May 15, 2019
- Simard, M.; Fatoyinbo, L.; Smetanka, C.; Rivera-Monroy, V. H.; Castañeda-Moya, E.; Thomas, N.; Van der Stocken, T. (2018). "Mangrove canopy height globally related to precipitation, temperature and cyclone frequency". Nature Geoscience. 12 (1): 40–45. doi:10.1038/s41561-018-0279-1. hdl:2060/20190029179. S2CID 134827807.
Further reading
- Saenger, Peter (2002). Mangrove Ecology, Silviculture, and Conservation. Kluwer Academic Publishers, Dordrecht. ISBN 1-4020-0686-1.
- Thanikaimoni, Ganapathi (1986). Mangrove Palynology UNDP/UNESCO and the French Institute of Pondicherry, ISSN 0073-8336 (E).
- Tomlinson, Philip B. (1986). The Botany of Mangroves. Cambridge University Press, Cambridge, ISBN 0-521-25567-8.
- Teas, H. J. (1983). Biology and Ecology of Mangroves. W. Junk Publishers, The Hague. ISBN 90-6193-948-8.
- Plaziat, Jean-Claude; Cavagnetto, Carla; Koeniguer, Jean-Claude; Baltzer, Frédéric (2001). "History and biogeography of the mangrove ecosystem, based on a critical reassessment of the paleontological record". Wetlands Ecology and Management. 9 (3): 161–180. doi:10.1023/A:1011118204434. S2CID 24980831.
- Jayatissa, L. P.; Dahdouh-Guebas, F.; Koedam, N. (2002). "A review of the floral composition and distribution of mangroves in Sri Lanka" (PDF). Botanical Journal of the Linnean Society. 138: 29–43. doi:10.1046/j.1095-8339.2002.00002.x.
- Ellison, Aaron M. (2000). "Mangrove Restoration: Do We Know Enough?". Restoration Ecology. 8 (3): 219–229. doi:10.1046/j.1526-100x.2000.80033.x.
- Agrawala, Shardul; Hagestad; Marca; Koshy, Kayathu; Ota, Tomoko; Prasad, Biman; Risbey, James; Smith, Joel; Van Aalst, Maarten. 2003. Development and Climate Change in Fiji: Focus on Coastal Mangroves. Organisation of Economic Co-operation and Development, Paris, Cedex 16, France.
- Barbier, E.B.; Sathirathai, S. (2001). "Valuing Mangrove Conservation in Southern Thailand". Contemporary Economic Policy. 19 (2): 109–122. doi:10.1111/j.1465-7287.2001.tb00054.x.
- Bosire, J.O.; Dahdouh-Guebas, F.; Jayatissa, L.P.; Koedam, N.; Lo Seen, D.; Nitto, Di D. (2005). "How Effective were Mangroves as a Defense Against the Recent Tsunami?". Current Biology. 15 (12): R443–R447. doi:10.1016/j.cub.2005.06.008. PMID 15964259. S2CID 8772526.
- Bowen, Jennifer L.; Valiela, Ivan; York, Joanna K. (2001). "Mangrove Forests: One of the World's Threatened Major Tropical Environments". BioScience. 51 (10): 807–815. doi:10.1641/0006-3568(2001)051[0807:mfootw]2.0.co;2.
- Jin-Eong, Ong (2004). "The Ecology of Mangrove Conservation and Management". Hydrobiologia. 295 (1–3): 343–351. doi:10.1007/BF00029141. S2CID 26686381.
- Glenn, C. R. 2006. "Earth's Endangered Creatures"
- Lewis, Roy R. III (2004). "Ecological Engineering for Successful Management and Restoration of Mangrove Forest". Ecological Engineering. 24 (4): 403–418. doi:10.1016/j.ecoleng.2004.10.003.
- Kuenzer, C.; Bluemel, A.; Gebhardt, S.; Vo Quoc, T. & Dech, S. (2011). "Remote Sensing of Mangrove Ecosystems: A Review". Remote Sensing. 3 (5): 878–928. Bibcode:2011RemS....3..878K. doi:10.3390/rs3050878.
- Lucien-Brun, H (1997). "Evolution of world shrimp production: Fisheries and aquaculture". World Aquaculture. 28: 21–33.
- Twilley, R. R., V.H. Rivera-Monroy, E. Medina, A. Nyman, J. Foret, T. Mallach, and L. Botero. 2000. Patterns of forest development in mangroves along the San Juan River estuary, Venezuela. Forest Ecology and Management
- Murray, M.R.; Zisman, S.A.; Furley, P.A.; Munro, D.M.; Gibson, J.; Ratter, J.; Bridgewater, S.; Mity, C.D.; Place, C.J. (2003). "The Mangroves of Belize: Part 1. Distribution, Composition and Classification". Forest Ecology and Management. 174 (1–3): 265–279. doi:10.1016/s0378-1127(02)00036-1.
- Vo Quoc, T.; Kuenzer, C.; Vo Quang, M.; Moder, F. & Oppelt, N. (December 2012). "Review of Valuation Methods for Mangrove Ecosystem Services". Ecological Indicators. 23: 431–446. doi:10.1016/j.ecolind.2012.04.022.
- Spalding, Mark; Kainuma, Mami and Collins, Lorna (2010) World Atlas of Mangroves Earthscan, London, ISBN 978-1-84407-657-4; 60 maps showing worldwide mangrove distribution
- Massó; Alemán, S.; Bourgeois, C.; Appeltans, W.; Vanhoorne, B.; De Hauwere, N.; Stoffelen, P.; Heaghebaert, A.; Dahdouh-Guebas, F. (2010). "The 'Mangrove Reference Database and Herbarium'" (PDF). Plant Ecology and Evolution. 143 (2): 225–232. doi:10.5091/plecevo.2010.439.
- Vo Quoc, T.; Oppelt, N.; Leinenkugel, P. & Kuenzer, C. (2013). "Remote Sensing in Mapping Mangrove Ecosystems – An Object-Based Approach". Remote Sensing. 5 (1): 183–201. Bibcode:2013RemS....5..183V. doi:10.3390/rs5010183.
External links
| Wikimedia Commons has media related to Mangrove. |
| Look up mangrove in Wiktionary, the free dictionary. |
- "Mangrove Factsheet". Waitt Institute. Archived from the original on 2015-09-04. Retrieved 2015-06-08.
- "Mangroves". Smithsonian Ocean Portal.
- "Mangroves Fact Sheet" (PDF). Fisheries Western Australia. 2013. Archived from the original (PDF) on April 23, 2013.* Rhizophoraceae at Curlie
- Mangrove forests at Curlie
- In May 2011, the VOA Special English service of the Voice of America broadcast a 15-minute program on mangrove forests. A transcript and MP3 of the program, intended for English learners, can be found at Mangrove Forests Could Be a Big Player in Carbon Trading
- "Water Center for the Humid Tropics of Latin America and the Caribbean". Archived from the original on 2012-02-05. Retrieved 2014-01-25.
- "Ocean Data Viewer – UNEP-WCMC". UNEP-WCMC's official website – Ocean Data Viewer. Retrieved 2020-11-27.


