Neopentyl glycol
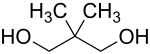
Neopentyl glycol (IUPAC name: 2,2-dimethylpropane-1,3-diol) is an organic chemical compound. It is used in the synthesis of polyesters, paints, lubricants, and plasticizers. When used in the manufacture of polyesters, it enhances the stability of the product towards heat, light, and water. By esterification reaction with fatty or carboxylic acids, synthetic lubricating esters with reduced potential for oxidation or hydrolysis, compared to natural esters, can be produced.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,2-Dimethylpropane-1,3-diol | |
| Other names
2,2-Dimethyl-1,3-propanediol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.347 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H12O2 | |
| Molar mass | 104.148 g/mol |
| Melting point | 129.13 °C (264.43 °F; 402.28 K) |
| Boiling point | 208 °C (406 °F; 481 K) |
| good | |
| Solubility | soluble in benzene, chloroform, very soluble in ethanol, diethyl ether |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
-551.2 kJ•mol−1 |
| Hazards | |
| Flash point | 129 °C (264 °F; 402 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Reactions
Neopentyl glycol is synthesized industrially by the aldol reaction of formaldehyde and isobutyraldehyde. This creates the intermediate hydroxypivaldehyde, which can be converted to neopentyl glycol with either excess formaldehyde or by palladium on carbon hydrogenation. [2]
It is used as a protecting group for ketones, for example in gestodene synthesis.
A condensation reaction of neopentyl glycol with 2,6-di-tert-butylphenol gives CGP-7930.
Organoboronic acid esters of neopentyl glycol are useful in the Suzuki reaction[3]
Research
It has been reported that plastic crystals of neopentyl glycol exhibit a colossal barocaloric effect (CBCEs), which is a cooling effect caused by pressure-induced phase transitions. The obtained entropy changes are about 389 joules per kilogram per kelvin near room temperature. This CBCE phenomenon is likely to be very useful in future solid-state refrigeration technologies.[4]
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 3‑228, 5‑42, 16‑22, ISBN 0-8493-0594-2
- Weissermel, Klaus; Arpe, Hans-Jürgen; Lindley, Charlet R. (2003), Industrial Organic Chemistry (4 ed.), Wiley-VCH, pp. 214–215, ISBN 978-3-527-30578-0, retrieved 2009-07-20
- Blair, D. J.; Zhong, S.; Hesse, M. J.; Zabaleta, N.; Myers, E. L.; Aggarwal, V. K. (2016). "Full chirality transfer in the synthesis of hindered tertiary boronic esters under in situ lithiation–borylation conditions". Chemical Communications. 52 (30): 5289–5292. doi:10.1039/C6CC00536E. ISSN 1359-7345.
- Li, Bing; et al. (27 March 2019), Nature, pp. 506–510
