Pegvaliase
Pegvaliase, sold under the brand name Palynziq, is a medication for the treatment of the genetic disease phenylketonuria.[1] Chemically, it is a pegylated derivative of the enzyme phenylalanine ammonia-lyase that metabolizes phenylalanine to reduce its blood levels.[2]
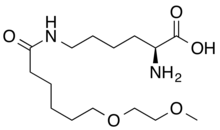 | |
| Clinical data | |
|---|---|
| Pronunciation | peg val' i ase |
| Trade names | Palynziq |
| Other names | Pegvaliase-pqpz; PEG-PAL; RAvPAL-PEG |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618057 |
| License data |
|
| Routes of administration | Subcutaneous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C15H30N2O5 |
| Molar mass | 318.414 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
It was approved by the Food and Drug Administration for use in the United States in 2018.[1] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[3]
References
- "FDA approves a new treatment for PKU, a rare and serious genetic disease" (Press release). Food and Drug Administration. May 24, 2018.
- "Palynziq". BioMarin Pharmaceutica.
- New Drug Therapy Approvals 2018 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2019. Retrieved 16 September 2020.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.