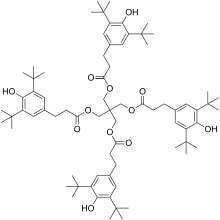
Pentaerythritol tetrakis(3,5-di-tert-butyl-4-hydroxyhydrocinnamate)
Pentaerythritol tetrakis(3,5-di-tert-butyl-4-hydroxyhydrocinnamate) is a chemical compound composed of 4 sterically hindered phenols linked through a pentaerythritol core. It is used as primary antioxidant for stabilizing polymers, particularly polyethylene and polypropylene.
 | |
| Names | |
|---|---|
| IUPAC name
Pentaerythritol tetrakis(3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate) | |
| Other names
Anox 20, Irganox 1010, Dovernox 10 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C73H108O12 | |
| Molar mass | 1177.655 g·mol−1 |
| Appearance | White solid |
| Melting point | 110–125 °C (230–257 °F; 383–398 K) |
| <0.1 g/ml | |
| Solubility in Acetone | 0.75 g/ml |
| Solubility in Toluene | 0.5 g/ml |
| Solubility in Methanol | <0.1 g/ml |
| Hazards | |
| H413 | |
| P273, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
It may be produced by the transesterification of 3-(3,5-ditert-butyl-4-hydroxyphenyl)propanoate esters with pentaerythritol.
Properties
The linking of phenols together with pentaerythritol maintains their activity with greatly reduced volatility. This is important during the processing and molding steps where the plastic is heated to molten, typically several hundred degrees.[1]
See also
References
- Vulic, Ivan; Vitarelli, Giacomo; Zenner, John M. (January 2002). "Structure–property relationships: phenolic antioxidants with high efficiency and low colour contribution". Polymer Degradation and Stability. 78 (1): 27–34. doi:10.1016/S0141-3910(02)00115-5.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
