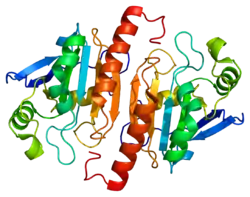
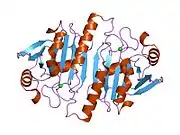
Peroxiredoxin 1
Peroxiredoxin-1 is a protein that in humans is encoded by the PRDX1 gene.[4][5]
Function
This gene encodes a member of the peroxiredoxin family of antioxidant enzymes, which reduce hydrogen peroxide and alkyl hydroperoxides.[6] The encoded protein may play an antioxidant protective role in cells, and may contribute to the antiviral activity of CD8(+) T-cells. This protein may have a proliferative effect and play a role in cancer development or progression. Three transcript variants encoding the same protein have been identified for this gene.[5]
Interactions
Peroxiredoxin 1 has been shown to interact with PRDX4.[7] A chemoproteomic approach has revealed that peroxiredoxin 1 is the main target of theonellasterone.[8]
Clinical significance
As enzymes that combat oxidative stress, peroxiredoxins play an important role in health and disease.[9] Peroxiredoxin 1 and peroxiredoxin 2 have been shown to be released by some cells when stimulated by LPS or TNF-alpha.[10] The released peroxiredoxin can then act to produce inflammatory cytokines.[10] The levels of peroxiredoxin 1 are elevated in pancreatic cancer and it can potentially act as a marker for the diagnosis and prognosis of this disease.[11] In some types of cancer, peroxiredoxin 1 has been determined to act as a tumor suppressor and other studies show that peroxiredoxin 1 is overexpressed in certain human cancers.[12] A recent study has found that peroxiredoxin 1 may play a role in tumorigenesis by regulating the mTOR/p70S6K pathway in esophageal squamous cell carcinoma.[12] The expression patterns of peroxiredoxin 1 along with peroxiredoxin 4 are involved in human lung cancer malignancy.[13] It has also been shown that peroxiredoxin 1 may be an important player in the pathogenesis of acute respiratory distress syndrome because of its role in promoting inflammation.[14]
References
- GRCh38: Ensembl release 89: ENSG00000117450 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Prospéri MT, Ferbus D, Karczinski I, Goubin G (May 1993). "A human cDNA corresponding to a gene overexpressed during cell proliferation encodes a product sharing homology with amoebic and bacterial proteins". The Journal of Biological Chemistry. 268 (15): 11050–6. PMID 8496166.
- "Entrez Gene: PRDX1 peroxiredoxin 1".
- Wu, C; Dai, H; Yan, L; Liu, T; Cui, C; Chen, T; Li, H (July 2017). "Sulfonation of the resolving cysteine in human peroxiredoxin 1: A comprehensive analysis by mass spectrometry". Free Radical Biology & Medicine. 108: 785–792. doi:10.1016/j.freeradbiomed.2017.04.341. PMC 5564515. PMID 28450148.
- Jin DY, Chae HZ, Rhee SG, Jeang KT (Dec 1997). "Regulatory role for a novel human thioredoxin peroxidase in NF-kappaB activation". The Journal of Biological Chemistry. 272 (49): 30952–61. doi:10.1074/jbc.272.49.30952. PMID 9388242.
- Margarucci L, Monti MC, Tosco A, Esposito R, Zampella A, Sepe V, Mozzicafreddo M, Riccio R, Casapullo A (Jan 2015). "Theonellasterone, a steroidal metabolite isolated from a Theonella sponge, protects peroxiredoxin-1 from oxidative stress reactions". Chemical Communications. 51 (9): 1591–3. doi:10.1039/c4cc09205h. PMID 25503482.
- El Eter E, Al-Masri AA (May 2015). "Peroxiredoxin isoforms are associated with cardiovascular risk factors in type 2 diabetes mellitus". Brazilian Journal of Medical and Biological Research. 48 (5): 465–9. doi:10.1590/1414-431X20144142. PMC 4445671. PMID 25742636.
- Mullen L, Hanschmann EM, Lillig CH, Herzenberg LA, Ghezzi P (2015). "Cysteine Oxidation Targets Peroxiredoxins 1 and 2 for Exosomal Release through a Novel Mechanism of Redox-Dependent Secretion". Molecular Medicine. 21: 98–108. doi:10.2119/molmed.2015.00033. PMC 4461588. PMID 25715249.
- Cai CY, Zhai LL, Wu Y, Tang ZG (Feb 2015). "Expression and clinical value of peroxiredoxin-1 in patients with pancreatic cancer". European Journal of Surgical Oncology. 41 (2): 228–35. doi:10.1016/j.ejso.2014.11.037. PMID 25434328.
- Gong F, Hou G, Liu H, Zhang M (Feb 2015). "Peroxiredoxin 1 promotes tumorigenesis through regulating the activity of mTOR/p70S6K pathway in esophageal squamous cell carcinoma". Medical Oncology. 32 (2): 455. doi:10.1007/s12032-014-0455-0. PMID 25579166.
- Jiang H, Wu L, Mishra M, Chawsheen HA, Wei Q (2014). "Expression of peroxiredoxin 1 and 4 promotes human lung cancer malignancy". American Journal of Cancer Research. 4 (5): 445–60. PMC 4163610. PMID 25232487.
- Liu D, Mao P, Huang Y, Liu Y, Liu X, Pang X, Li Y (2014). "Proteomic analysis of lung tissue in a rat acute lung injury model: identification of PRDX1 as a promoter of inflammation". Mediators of Inflammation. 2014: 1–14. doi:10.1155/2014/469358. PMC 4082880. PMID 25024510.
Further reading
- Wood ZA, Schröder E, Robin Harris J, Poole LB (Jan 2003). "Structure, mechanism and regulation of peroxiredoxins". Trends in Biochemical Sciences. 28 (1): 32–40. doi:10.1016/S0968-0004(02)00003-8. PMID 12517450.
- Sauri H, Butterfield L, Kim A, Shau H (Mar 1995). "Antioxidant function of recombinant human natural killer enhancing factor". Biochemical and Biophysical Research Communications. 208 (3): 964–9. doi:10.1006/bbrc.1995.1428. PMID 7702627.
- Shau H, Butterfield LH, Chiu R, Kim A (1994). "Cloning and sequence analysis of candidate human natural killer-enhancing factor genes". Immunogenetics. 40 (2): 129–34. doi:10.1007/BF00188176. PMID 8026862.
- Kawai S, Takeshita S, Okazaki M, Kikuno R, Kudo A, Amann E (Apr 1994). "Cloning and characterization of OSF-3, a new member of the MER5 family, expressed in mouse osteoblastic cells". Journal of Biochemistry. 115 (4): 641–3. doi:10.1093/oxfordjournals.jbchem.a124388. PMID 8089076.
- Shau H, Kim A (Feb 1994). "Identification of natural killer enhancing factor as a major antioxidant in human red blood cells". Biochemical and Biophysical Research Communications. 199 (1): 83–8. doi:10.1006/bbrc.1994.1197. PMID 8123050.
- Prospéri MT, Apiou F, Dutrillaux B, Goubin G (Jan 1994). "Organization and chromosomal assignment of two human PAG gene loci: PAGA encoding a functional gene and PAGB a processed pseudogene". Genomics. 19 (2): 236–41. doi:10.1006/geno.1994.1053. PMID 8188254.
- Wen ST, Van Etten RA (Oct 1997). "The PAG gene product, a stress-induced protein with antioxidant properties, is an Abl SH3-binding protein and a physiological inhibitor of c-Abl tyrosine kinase activity". Genes & Development. 11 (19): 2456–67. doi:10.1101/gad.11.19.2456. PMC 316562. PMID 9334312.
- Jin DY, Chae HZ, Rhee SG, Jeang KT (Dec 1997). "Regulatory role for a novel human thioredoxin peroxidase in NF-kappaB activation". The Journal of Biological Chemistry. 272 (49): 30952–61. doi:10.1074/jbc.272.49.30952. PMID 9388242.
- Outinen PA, Sood SK, Pfeifer SI, Pamidi S, Podor TJ, Li J, Weitz JI, Austin RC (Aug 1999). "Homocysteine-induced endoplasmic reticulum stress and growth arrest leads to specific changes in gene expression in human vascular endothelial cells". Blood. 94 (3): 959–67. doi:10.1182/blood.V94.3.959.415k20_959_967. PMID 10419887.
- Yanagawa T, Ishikawa T, Ishii T, Tabuchi K, Iwasa S, Bannai S, Omura K, Suzuki H, Yoshida H (Oct 1999). "Peroxiredoxin I expression in human thyroid tumors". Cancer Letters. 145 (1–2): 127–32. doi:10.1016/S0304-3835(99)00243-8. PMID 10530780.
- Noh DY, Ahn SJ, Lee RA, Kim SW, Park IA, Chae HZ (2001). "Overexpression of peroxiredoxin in human breast cancer". Anticancer Research. 21 (3B): 2085–90. PMID 11497302.
- Xu XR, Huang J, Xu ZG, Qian BZ, Zhu ZD, Yan Q, Cai T, Zhang X, Xiao HS, Qu J, Liu F, Huang QH, Cheng ZH, Li NG, Du JJ, Hu W, Shen KT, Lu G, Fu G, Zhong M, Xu SH, Gu WY, Huang W, Zhao XT, Hu GX, Gu JR, Chen Z, Han ZG (Dec 2001). "Insight into hepatocellular carcinogenesis at transcriptome level by comparing gene expression profiles of hepatocellular carcinoma with those of corresponding noncancerous liver". Proceedings of the National Academy of Sciences of the United States of America. 98 (26): 15089–94. Bibcode:2001PNAS...9815089X. doi:10.1073/pnas.241522398. PMC 64988. PMID 11752456.
- Kim SH, Fountoulakis M, Cairns N, Lubec G (2002). Protein levels of human peroxiredoxin subtypes in brains of patients with Alzheimer's disease and Down syndrome. Journal of Neural Transmission. Supplementum. pp. 223–35. doi:10.1007/978-3-7091-6262-0_18. ISBN 978-3-211-83704-7. PMID 11771746.
- Rabilloud T, Heller M, Gasnier F, Luche S, Rey C, Aebersold R, Benahmed M, Louisot P, Lunardi J (May 2002). "Proteomics analysis of cellular response to oxidative stress. Evidence for in vivo overoxidation of peroxiredoxins at their active site". The Journal of Biological Chemistry. 277 (22): 19396–401. doi:10.1074/jbc.M106585200. PMID 11904290.
- Chang TS, Jeong W, Choi SY, Yu S, Kang SW, Rhee SG (Jul 2002). "Regulation of peroxiredoxin I activity by Cdc2-mediated phosphorylation". The Journal of Biological Chemistry. 277 (28): 25370–6. doi:10.1074/jbc.M110432200. PMID 11986303.
- Wagner E, Luche S, Penna L, Chevallet M, Van Dorsselaer A, Leize-Wagner E, Rabilloud T (Sep 2002). "A method for detection of overoxidation of cysteines: peroxiredoxins are oxidized in vivo at the active-site cysteine during oxidative stress". The Biochemical Journal. 366 (Pt 3): 777–85. doi:10.1042/BJ20020525. PMC 1222825. PMID 12059788.
- Shen C, Nathan C (Feb 2002). "Nonredundant antioxidant defense by multiple two-cysteine peroxiredoxins in human prostate cancer cells". Molecular Medicine. 8 (2): 95–102. doi:10.1007/BF03402079. PMC 2039972. PMID 12080185.
- Yang KS, Kang SW, Woo HA, Hwang SC, Chae HZ, Kim K, Rhee SG (Oct 2002). "Inactivation of human peroxiredoxin I during catalysis as the result of the oxidation of the catalytic site cysteine to cysteine-sulfinic acid". The Journal of Biological Chemistry. 277 (41): 38029–36. doi:10.1074/jbc.M206626200. PMID 12161445.
- Geiben-Lynn R, Kursar M, Brown NV, Addo MM, Shau H, Lieberman J, Luster AD, Walker BD (Jan 2003). "HIV-1 antiviral activity of recombinant natural killer cell enhancing factors, NKEF-A and NKEF-B, members of the peroxiredoxin family". The Journal of Biological Chemistry. 278 (3): 1569–74. doi:10.1074/jbc.M209964200. PMID 12421812.