Plasmodium berghei
Plasmodium berghei is a species in the genus Plasmodium subgenus Vinckeia.
| Plasmodium berghei | |
|---|---|
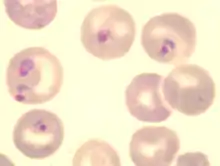 | |
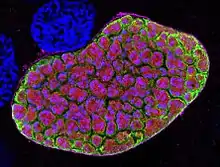
| Blood forms of the rodent malaria parasite Plasmodium berghei | |
| Scientific classification | |
| (unranked): | Diaphoretickes |
| Clade: | TSAR |
| Clade: | SAR |
| Infrakingdom: | Alveolata |
| Phylum: | Apicomplexa |
| Class: | Aconoidasida |
| Order: | Haemospororida |
| Family: | Plasmodiidae |
| Genus: | Plasmodium |
| Species: | P. berghei |
| Binomial name | |
| Plasmodium berghei Vincke & Lips, 1948 | |
It is a protozoan parasite that causes malaria in certain rodents. Originally, isolated from thicket rats in Central Africa, P. berghei is one of four Plasmodium species that have been described in African murine rodents, the others being Plasmodium chabaudi, Plasmodium vinckei, and Plasmodium yoelii. Due to its ability to infect rodents and relative ease of genetic engineering, P. berghei is a popular model organism for the study of human malaria.
Biology
Like all malaria parasites of mammals, including the four human malaria parasites, P. berghei is transmitted by Anopheles mosquitoes and it infects the liver after being injected into the bloodstream by a bite of an infected female mosquito. After a short period (a few days) of development and multiplication, these parasites leave the liver and invade erythrocytes (red blood cells). The multiplication of the parasite in the blood causes the pathology such as anaemia and damage of essential organs of the host such as lungs, liver, spleen. P. berghei infections may also affect the brain and can be the cause of cerebral complications in laboratory mice. These symptoms are to a certain degree comparable to symptoms of cerebral malaria in patients infected with the human malaria parasite Plasmodium falciparum.[1]
Distribution
Plasmodium berghei is found in the forests of Central Africa, where its natural cyclic hosts are the thicket rat (Grammomys surdaster) and the mosquito (Anopheles dureni).
Hosts
Plasmodium berghei was first identified in the thicket rat (Grammomys surdaster). It has also been described in Leggada bella, Praomys jacksoni and Thamnomys surdaster. In research laboratories, various rodents can be infected, such as mice, rats and gerbils (Meriones unguiculatus).[2]
The natural insect host of P. berghei is likely Anopheles dureni, however in laboratory conditions it has also been shown to infect Anopheles stephensi.
History
This species was first described by Vincke and Lips in 1948 in the Belgian Congo.[3]
Research
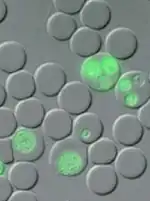
Plasmodium berghei infection of laboratory mouse strains is frequently used in research as a model for human malaria.[4] In the laboratory the natural hosts have been replaced by a number of commercially available laboratory mouse strains, and the mosquito Anopheles stephensi, which is comparatively easily reared and maintained under defined laboratory conditions.
Plasmodium berghei is used as a model organism for the investigation of human malaria because of its similarity to the Plasmodium species which cause human malaria. P. berghei has a very similar life-cycle to the species that infect humans, and it causes disease in mice which has signs similar to those seen in human malaria. Importantly, P. berghei can be genetically manipulated more easily than the species which infect humans, making it a useful model for research into Plasmodium genetics.
In several aspects the pathology caused by P. berghei in mice differs from malaria caused by P. falciparum in humans. In particular, while death from P. falciparum malaria in humans is most frequently caused by the accumulation of red blood cells in the blood vessels of the brain, it is unclear to what extent this occurs in mice infected with P. berghei.[4] Instead, in P. berghei infection, mice are found to have an accumulation of immune cells in brain blood vessels.[4] This has led some to question the use of P. berghei infections in mice as an appropriate model of cerebral malaria in humans.[4]
Plasmodium berghei can be genetically manipulated in the laboratory using standard genetic engineering technologies. Consequently, this parasite is often used for the analysis of the function of malaria genes using the technology of genetic modification.[5][6][7] Additionally, the genome of P. berghei has been sequenced and it shows a high similarity, both in structure and gene content, with the genome of the human malaria parasite Plasmodium falciparum.[8][9][10]
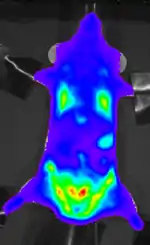
A number of genetically modified P. berghei lines have been generated which express fluorescent reporter proteins such as Green Fluorescent Protein (GFP) and mCherry (red) or bioluminescent reporters such as Luciferase. These transgenic parasites are important tools to study and visualize the parasites in the living host.[11][12]
P. berghei is used in research programs for development and screening of anti-malarial drugs and for the development of an effective vaccine against malaria.[13]
References
- Franke-Fayard B, et al. (2010). "Sequestration and tissue accumulation of human malaria parasites: can we learn anything from rodent models of malaria?". PLoS Pathogens. 6 (9): e1001032. doi:10.1371/journal.ppat.1001032. PMC 2947991. PMID 20941396.
- Junaid, Quazim Olawale; Khaw, Loke Tim; Mahmud, Rohela; Ong, Kien Chai; Lau, Yee Ling; Borade, Prajakta Uttam; Liew, Jonathan Wee Kent; Sivanandam, Sinnadurai; Wong, Kum Thong; Vythilingam, Indra (2017). "Pathogenesis of Plasmodium berghei ANKA infection in the gerbil (Meriones unguiculatus) as an experimental model for severe malaria". Parasite. 24: 38. doi:10.1051/parasite/2017040. PMC 5642054. PMID 29034874.

- Vincke, I.H. and Lips, M. (1948) Un nouveau plasmodium d'un rongeur sauvage du Congo: Plasmodium berghei n.sp. Annales de la Société Belge de Médecine Tropicale 28, 97-104
- Craig AG; Grau GE; Janse C; Kazura JW; Milner D; Barnwell JW; Turner G; Langhorne J (February 2012). "The Role of Animal Models for Research on Severe Malaria". PLOS Pathogens. 8 (2): e1002401. doi:10.1371/journal.ppat.1002401. PMC 3271056. PMID 22319438.
- Janse CJ; Ramesar J; Waters AP (2006). "High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei". Nature Protocols. 1 (1): 346–56. doi:10.1038/nprot.2006.53. PMID 17406255.
- Janse C.J.; et al. (2011). "A genotype and phenotype database of genetically modified malaria-parasites". Trends Parasitol. 27 (1): 31–39. doi:10.1016/j.pt.2010.06.016. PMID 20663715.
- Khan SM; Kroeze H; Franke-Fayard B; Janse CJ (2013). Standardization in generating and reporting genetically modified rodent malaria parasites: the RMgmDB database. Methods Mol Biol. Methods in Molecular Biology. 923. pp. 139–50. doi:10.1007/978-1-62703-026-7_9. ISBN 978-1-62703-025-0. PMID 22990775.
- Hall; et al. (2005). "A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses". Science. 307 (5706): 82–6. Bibcode:2005Sci...307...82H. doi:10.1126/science.1103717. PMID 15637271.
- Kooij TW; Janse CJ; Waters AP (2006). "Plasmodium post-genomics: better the bug you know?". Nat Rev Microbiol. 4 (5): 344–357. doi:10.1038/nrmicro1392. PMID 16582929.
- Otto TD; et al. (2014). "A comprehensive evaluation of rodent malaria parasite genomes and gene expression". BMC Biology. 12: 86. doi:10.1186/s12915-014-0086-0. PMC 4242472. PMID 25359557.
- Amino R, Ménard R, Frischknecht F (2005). "In vivo imaging of malaria parasites--recent advances and future directions". Curr Opin Microbiol. 8 (4): 407–14. doi:10.1016/j.mib.2005.06.019. PMID 16019254.
- Franke-Fayard B, Waters AP, Janse CJ (2006). "Real-time in vivo imaging of transgenic bioluminescent blood stages of rodent malaria parasites in mice". Nature Protocols. 1 (1): 476–85. doi:10.1038/nprot.2006.69. PMID 17406270.
- Khan SM, Janse CJ, Kappe SH, Mikolajczak SA (2012). "Genetic engineering of attenuated malaria parasites for vaccination". Curr Opin Biotechnol. 23 (6): 908–916. doi:10.1016/j.copbio.2012.04.003. PMID 22560204.
External links
| Wikimedia Commons has media related to Plasmodium berghei. |
- General information about (the biology of) P. berghei
- Information about the genome and genes of P. berghei