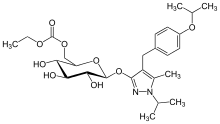
Remogliflozin etabonate
Remogliflozin etabonate (INN/USAN)[1] is a drug of the gliflozin class for the treatment of non-alcoholic steatohepatitis ("NASH") and type 2 diabetes. Remogliflozin was discovered by the Japanese company Kissei Pharmaceutical and is currently being developed by BHV Pharma, a wholly owned subsidiary of North Carolina USA-based Avolynt, and Glenmark Pharmaceuticals through a collaboration with BHV.[2] Remogliflozin was commercially launched first in India by Glenmark in May 2019.
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C26H38N2O9 |
| Molar mass | 522.595 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Clinical trials
Remogliflozin etabonate was shown to enhance urinary glucose excretion in rodents and humans. Early studies in diabetics improved plasma glucose levels.[3][4] Remogliflozin etabonate has been studied at doses up to 1000 mg.[5] A pair of 12-week phase 2b randomized clinical trials of diabetics published in 2015, found reductions in glycated hemoglobin and that it was generally well tolerated.[6]
Method of action
Remogliflozin etabonate is a pro-drug of remogliflozin. Remogliflozin inhibits the sodium-glucose transport proteins (SGLT), which are responsible for glucose reabsorption in the kidney. Blocking this transporter causes blood glucose to be eliminated through the urine.[7] Remogliflozin is selective for SGLT2.
See also
References
- Statement on a nonproprietory name adopted by the USAN council
- "Avolynt Announces Completion of Phase 2b BRID Study of SGLT2 Inhibitor Remogliflozin-Etabonate" (Press release). Avolynt, Inc. Retrieved July 24, 2018.
- Mudaliar S, Armstrong DA, Mavian AA, O'Connor-Semmes R, Mydlow PK, Ye J, et al. (November 2012). "Remogliflozin etabonate, a selective inhibitor of the sodium-glucose transporter 2, improves serum glucose profiles in type 1 diabetes". Diabetes Care. 35 (11): 2198–200. doi:10.2337/dc12-0508. PMC 3476920. PMID 23011728.
- Dobbins RL, O'Connor-Semmes R, Kapur A, Kapitza C, Golor G, Mikoshiba I, et al. (January 2012). "Remogliflozin etabonate, a selective inhibitor of the sodium-dependent transporter 2 reduces serum glucose in type 2 diabetes mellitus patients". Diabetes, Obesity & Metabolism. 14 (1): 15–22. doi:10.1111/j.1463-1326.2011.01462.x. PMID 21733056. S2CID 23372554.
- Sykes AP, O'Connor-Semmes R, Dobbins R, Dorey DJ, Lorimer JD, Walker S, et al. (January 2015). "Randomized trial showing efficacy and safety of twice-daily remogliflozin etabonate for the treatment of type 2 diabetes". Diabetes, Obesity & Metabolism. 17 (1): 94–7. doi:10.1111/dom.12391. PMID 25223369. S2CID 6436562.
- Sykes AP, Kemp GL, Dobbins R, O'Connor-Semmes R, Almond SR, Wilkison WO, et al. (January 2015). "Randomized efficacy and safety trial of once-daily remogliflozin etabonate for the treatment of type 2 diabetes". Diabetes, Obesity & Metabolism. 17 (1): 98–101. doi:10.1111/dom.12393. PMID 25238025. S2CID 25280330.
- "Molecule of the Month: Dapagliflozin". Prous Science. November 2007. Archived from the original on January 6, 2008.