Risdiplam
Risdiplam, sold under the brand name Evrysdi, is a medication used to treat spinal muscular atrophy (SMA)[2][3] and the first oral medication approved to treat this disease.[2][3]
 | |
| Clinical data | |
|---|---|
| Trade names | Evrysdi |
| Other names | RG7916; RO7034067 |
| AHFS/Drugs.com | Monograph |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
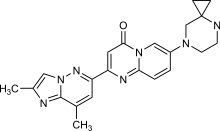
| Formula | C22H23N7O |
| Molar mass | 401.474 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Risdiplam is a survival of motor neuron 2-directed RNA splicing modifier.[2][1][4]
The most common side effects include fever, diarrhea, rash, ulcers of the mouth area, joint pain (arthralgia) and urinary tract infections.[2][1] Additional side effects for the infantile-onset population include upper respiratory tract infection, pneumonia, constipation and vomiting.[2][1]
It was approved by the US Food and Drug Administration (FDA) in August 2020, for the treatment of adults and children two months of age or older.[2][5] Developed in association with PTC Therapeutics and the SMA Foundation,[3][5] it is marketed in the US by Genentech,[2] a subsidiary of Roche.[5]
Medical uses
In the United States, risdiplam is indicated to treat people two months of age and older with spinal muscular atrophy.[2][1]
Adverse effects
The most common side effects include fever, diarrhoea, rash, ulcers of the mouth area, joint pain (arthralgia) and urinary tract infections.[2][1] Additional side effects for the infantile-onset population include upper respiratory tract infection, pneumonia, constipation and vomiting.[2][1]
Risdiplam should not be taken together with medications that are multidrug and toxin extrusion (MATE) substrates because risdiplam may increase plasma concentrations of these drugs.[2][1]
Pharmacology
Mechanism of action
Risdiplam addresses the underlying cause of SMA: a reduced amount of survival motor neuron (SMN) protein. The protein is encoded by the SMN1 and SMN2 genes. SMA is caused by mutations in SMN1 that code for inactive forms of the protein. The activity of the SMN2 gene, which produces much smaller quantities of SMN, tends to determine the severity of disease.[3][6]
The compound is a pyridazine derivative that modifies the splicing of SMN2 messenger RNA,[7][4] resulting in up to a 2-fold increase in the concentration of the functional SMN protein in vivo.[8]
Nusinersen, the first drug approved to treat SMA, works in a similar way.[9]
Efficacy
The safety and efficacy of risdiplam in infantile-onset and later-onset SMA has been evaluated in two ongoing clinical trials.[3][10][11]
In the infantile-onset SMA study, an open-label trial with 41 participants, efficacy was established based on the ability to sit without support for at least five seconds. After 12 months of treatment, 29% of participants were able to sit independently for more than five seconds. After 23 or more months of treatment, 81% of participants were alive without permanent ventilation. Although the study did not perform direct comparisons against children receiving a placebo (inactive treatment), these results compare favourably with the typical course of the untreated disease.[10][2]
The study of later-onset SMA was a randomised controlled trial that enrolled 180 participants, aged between 2 and 25 years, with less severe forms of the disease. Participants treated with risdiplam for 12 months showed improvements in motor function compared to participants given a placebo.[11][2][3]
Two further clinical studies are underway as of August 2020.[3][5]
Society and culture
Legal status
The US Food and Drug Administration (FDA) awarded marketing approval to Genentech on August 7, 2020. The FDA earlier granted the application for risdiplam fast track, priority review, and orphan drug designations.[2][3][5] Genentech was also awarded a rare pediatric disease priority review voucher.[2]
The European Medicines Agency (EMA) awarded risdiplam a priority medicine designation in 2018[5][12][13] and an orphan drug designation in 2019.[14][5]
As of August 2020, Roche has applied for marketing authorisation in Brazil, Chile, China, the European Union, Indonesia, Russia, South Korea and Taiwan.[5][15]
Names
Risdiplam is the International nonproprietary name (INN).[16]
Compassionate use
Since late 2019, Roche has been offering the drug globally for free for eligible people through an expanded access program.[17]
References
- "Evrysdi- risdiplam powder, for solution". DailyMed. 18 August 2020. Retrieved 24 September 2020.
- "FDA Approves Oral Treatment for Spinal Muscular Atrophy". U.S. Food and Drug Administration (FDA) (Press release). 7 August 2020. Retrieved 7 August 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Evrysdi (Risdiplam)". smanewstoday.com. 7 August 2020. Retrieved 8 August 2020.
- Zhao X, Feng Z, Ling KK, Mollin A, Sheedy J, Yeh S, et al. (May 2016). "Pharmacokinetics, pharmacodynamics, and efficacy of a small-molecule SMN2 splicing modifier in mouse models of spinal muscular atrophy". Human Molecular Genetics. 25 (10): 1885–99. doi:10.1093/hmg/ddw062. PMC 5062580. PMID 26931466.
- "FDA Approves Genentech's Evrysdi (risdiplam) for Treatment of Spinal Muscular Atrophy (SMA) in Adults and Children 2 Months and Older". Genentech (Press release). 7 August 2020. Retrieved 7 August 2020.
- Ramdas, Sithara; Servais, Laurent (24 January 2020). "New treatments in spinal muscular atrophy: an overview of currently available data". Expert Opinion on Pharmacotherapy. Informa UK Limited. 21 (3): 307–315. doi:10.1080/14656566.2019.1704732. ISSN 1465-6566. PMID 31973611. S2CID 210880199.
- Maria Joao Almeida (2016-09-08). "RG7916". BioNews Services. Retrieved 2017-10-08.
- Ratni, Hasane; Ebeling, Martin; Baird, John; Bendels, Stefanie; Bylund, Johan; Chen, Karen S.; Denk, Nora; Feng, Zhihua; Green, Luke; Guerard, Melanie; Jablonski, Philippe; Jacobsen, Bjoern; Khwaja, Omar; Kletzl, Heidemarie; Ko, Chien-Ping; Kustermann, Stefan; Marquet, Anne; Metzger, Friedrich; Mueller, Barbara; Naryshkin, Nikolai A.; Paushkin, Sergey V.; Pinard, Emmanuel; Poirier, Agnès; Reutlinger, Michael; Weetall, Marla; Zeller, Andreas; Zhao, Xin; Mueller, Lutz (25 July 2018). "Discovery of Risdiplam, a Selective Survival of Motor Neuron-2 (SMN2) Gene Splicing Modifier for the Treatment of Spinal Muscular Atrophy (SMA)". Journal of Medicinal Chemistry. American Chemical Society (ACS). 61 (15): 6501–6517. doi:10.1021/acs.jmedchem.8b00741. ISSN 0022-2623. PMID 30044619.
- Zanetta C, Nizzardo M, Simone C, Monguzzi E, Bresolin N, Comi GP, et al. (January 2014). "Molecular therapeutic strategies for spinal muscular atrophies: current and future clinical trials". Clinical Therapeutics. 36 (1): 128–40. doi:10.1016/j.clinthera.2013.11.006. PMID 24360800.
- Baranello G, Servais L, Day J, Deconinck N, Mercuri E, Klein A, et al. (October 2019). "P.353FIREFISH Part 1: 16-month safety and exploratory outcomes of risdiplam (RG7916) treatment in infants with type 1 spinal muscular atrophy". Neuromuscular Disorders. 29: S184. doi:10.1016/j.nmd.2019.06.515. ISSN 0960-8966.
- Mercuri E, Baranello G, Kirschner J, Servais L, Goemans N, Pera MC, et al. (April 2019). "Update from SUNFISH Part 1: Safety, Tolerability and PK/PD from the Dose-Finding Study, Including Exploratory Efficacy Data in Patients with Type 2 or 3 Spinal Muscular Atrophy (SMA) Treated with Risdiplam (RG7916) (S25.007)". Neurology. 92 (15 Supplement). ISSN 0028-3878.
- Inacio P (2018-12-21). "Risdiplam Granted EMA's PRIME Designation for Potential in Spinal Muscular Atrophy". SMA News Today. Retrieved 8 August 2020.
- "PRIME designation granted by European Medicines Agency for Roche's risdiplam for treatment of spinal muscular atrophy (SMA)". Roche (Press release). 17 December 2018. Retrieved 12 August 2020.
- "EU/3/19/2145". European Medicines Agency (EMA). 9 April 2019. Retrieved 12 August 2020.
- "PTC Announces the Acceptance of the European Marketing Authorization Application for Evrysdi (risdiplam) for the Treatment of Spinal Muscular Atrophy". PTC Therapeutics, Inc. Retrieved 2020-08-18.
- World Health Organization (2018). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 80". WHO Drug Information. 32 (3): 482. hdl:10665/330907.
- "Roche announces global compassionate use programme for risdiplam". Spinal Muscular Atrophy UK. Retrieved 2020-04-08.
External links
- "Risdiplam". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT02913482 for "Investigate Safety, Tolerability, PK, PD and Efficacy of Risdiplam (RO7034067) in Infants With Type1 Spinal Muscular Atrophy (FIREFISH)" at ClinicalTrials.gov
- Clinical trial number NCT02908685 for "A Study to Investigate the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics and Efficacy of Risdiplam (RO7034067) in Type 2 and 3 Spinal Muscular Atrophy (SMA) Participants (SUNFISH)" at ClinicalTrials.gov