Rotzo Formation
The Rotzo Formation is a geological formation in Italy, dating to roughly between 189 and 183 million years ago and covering the Pliensbachian stage of the Jurassic Period in the Mesozoic Era.[2] Has been traditionally classified as a Sinemurian-Pliensbachian Formation, but a large and detailed dataset of isotopic 13C and 87Sr/86Sr data, estimated the Rotzo Formation to span only over the whole Pliensbachian.[3]
| Rotzo Formation Stratigraphic range: Early-late Pliensbachian ~184 Ma | |
|---|---|
 Exposed layer | |
| Type | Geological formation |
| Unit of | Calcari Grigi Group |
| Sub-units | Bellori outcrop, Monte Pasubio, Roverè di Velo Tracksites: Marocche di Dro, Coste dell'Anglone, Bella Lasta |
| Underlies | Calcari Grigi di Noriglio Formation |
| Overlies | Monte Zugna Formation |
| Lithology | |
| Primary | Lagoonal or restricted shallow subtidal; lithified, gray, silty marl. Paralic; ooidal, gray grainstone and bioturbated, intraclastic, ooidal, gray wackestone. Subtidal flat with mud banks and sand deposits.[1] |
| Other | Light-grey to yellowish-grey packstone, with oolites, bioclasts, algal lumps, pellets, dasycladacean algae, foraminifera, lituolids, and miliolids |
| Location | |
| Coordinates | 45.7°N 11.1°E |
| Approximate paleocoordinates | 32.1°S 16.7°E |
| Region | Veneto |
| Country | |
| Type section | |
| Named for | Rotzo |
 Rotzo Formation (Italy) | |
Fossil prosauropod tracks have been reported from the formation.[4] This formation was deposited within a tropical lagoon environment which was protected by oolitic shoals and bars from the open deep sea located to the east (Belluno Basin) and towards the west (Lombardia Basin). It is characterized by a rich paleontological content. It is notable mostly thanks to its great amount of big aberrant bivalves, among which is the genus Lithiotis, described in the second half of the nineteenth century. The unusual shape of Lithiotis and Cochlearites shells, extremely elongated and narrow, characterized by a spoon-like body space placed in a high position, rarely preserved, seems to suggest their adaptation to soft and muddy bottoms with a high sedimentation rate.[5] The Bellori outcrop displays about 20 m of limestones with intercalated clays and marls rich in organic matter and sometimés fossil wood (coal) and amber. The limestones are well stratified, with beds 10 cm to more than one metre thick, whereas the clayey levels range between 3 and 40 cm in thickness.[6][7]
Invertebrata
Microfossils of the Rotzo Formation consist of benthic foraminifera, calcareous algae, Ostracoda and coprolites. Foraminifera are mainly benthic agglutinated species belonging to the superfamily Lituolacea (suborder Textulariina), while lamellar and porcellaneous-walled species are very rare.[8] The bivalve Opisoma excavatum is very common.[9]
Ichnofossils
In the Western Venetian Prealps a shallow-water, oceanic carbonate platform system, the Trento platform, developed on the Early Jurassic, producing a large succession of massive to well-bedded white Limestones, several 100 m thick that are part of the Calcari Grigi Group, where the Rotzo Formation is the Upper Member.[10] On the local limestone of the Rotzo Formation deep burrowing is a very common type of biogenic activity, as is shown due to the presence of a large characteristic network of burrows which reach down to the lagoonal, marly-clayey assigned strata, suggesting intense bioturbation by large unknown organisms, perhaps giant decapod crustaceans (Probably members of the family Erymidae), although, the burrows found aren't closely related to the ones of Shrimps or other decapods, but resemble those of Stomatopoda and Malacostraca.[10] Other includes abandoned burrows, vertical biogenic action and infilling on the sea substrate.[10]
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
Campomolon, Valbona |
Burrowing and track Ichnofossils |
Thalassinoides suevicus has been found on mostly of the middle-upper part of the Rotzo Formation associated with muddy deposits. It ranges from 2–5 cm to 6–10 cm and the larger ones from 10–16 cm.[11] Y-shaped tunnels that seen in cross-section reveal circular walls made of pelletoidal grainstone, being more probably a fodichnia of a burrowing animal.[13] A few ichnofossils include simple cylindrical tubes up to 80 cm in length, that resemble crustacean described in Seychelles.[13] |
.jpg.webp) Thalassinoides | |
|
Campomolon, Valbona |
Burrowing and track Ichnofossils |
Two major types of Ophiomorpha where recovered, a smaller one from 2–4 cm in size and the larger one from 5–15 cm in diameter.[13] They are complex burrow systems lined with pelletoidal sediments generally infilled by coarse-grained detritus.[11] isp. A seem partly destroyed by weathering.[12] |
 Ophiomorpha | |
|
Campomolon, Valbona |
Burrowing and track Ichnofossils |
In the Rotzo Formation Ophiomorpha irregulaire local specimens the walls are extensively reworked by small, secondary burrowers assigned to the ichnogenus Chondrites.[12] Interpreted as the feeding burrow of a sediment-ingesting animal. |
 Chondrites | |
|
Campomolon, Valbona |
Infilled abandoned burrows by coarse-grained skelletal debris |
Ichnofossils done by organisms advancing along the bottom surface. Very narrow, vertical or subvertical, slightly winding unlined shafts filled with mud. Locally, post hurricane burrows are found in fine-grained tempestite beds and muddy layers and they are Domichnia, Fodinichnia and Chemichnia.[10] |
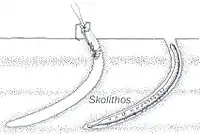 Skolithos | |
|
Glossifungites[10] |
|
Campomolon, Valbona |
Infilled abandoned burrows by coarse-grained skelletal debris |
On the local waters during the Lower Jurassic, water motion due to the hurricane action truncated many mounds causing changes on the deposition on the sea-floor and inducing various phases of substrate infillings with carbonate mud, fine-to coarse-grained skeletal debris and fecal pellets.[10] They are assigned to Priapulida, Serpulidae, Siboglinidae, Sabellidae or even Oweniidae. |
|
|
Chomatichnus[10] |
|
Campomolon, Valbona |
Vertical burrows with preserved entrances |
It is difficult to suggest this ichnogenus because on the Formation the vertical and lined burrow with a deep central crater typical of Chomatichnus is never preserved.[10] It resemble described burrows of endobenthic thalassinidean decapods, specially Callianassa subterranea of modern North Sea, Callianassa major, Callianassa californensis or Upogebia pugettensis.[10] It can be also Serpulidae Polychaetan burrows. |
|
Ammonoidea
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
Juraphyllites libertus |
Contrada Ronchi (Recoaro Terme, Vicenza) |
Shells of different sizes.[15] |
Type member of the family Juraphyllitidae. It is the most abundant Ammonite found on the Rotzo Formation |
 Juraphyllites (G) | |
|
|
Shells of different sizes.[15] |
An Ammonite of the Family Hildoceratidae |
 Fuciniceras | |
|
Protogrammoceras[16] |
Protogrammoceras sp. |
|
Shells of different sizes. |
An Ammonite of the family Hildoceratidae. |
|
|
Ugdulenaia cf. ugdulenai |
|
Shells of different sizes. |
An Ammonite of the family Hildoceratidae. |
||
|
Partschiceras[16] |
Partschiceras anonimum |
|
Shells of different sizes. |
An Ammonite of the family Phylloceratidae. |
|
|
Charmasseiceras sp. |
Shells of different sizes.[15] |
An Ammonite of the family Schlotheimiidae. A very rare genus on the layers of the formation, being found only a few specimens. |
|||
Gasteropoda
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
Neritopsis fabianii |
Certosa di Vedana |
Shells of different sizes.[18] |
A Marine Gasteropod (Snail), type genus of the Family Neritopsidae inside Cycloneritimorpha. |
||
|
Guidonia[18] |
Guidonia pseudorotula |
Certosa di Vedana |
Shells of different sizes.[18] |
A Marine Gasteropod (Snail) of the Family Trochonematidae inside Murchisoniina. |
|
|
Pseudorhytidopilus[18] |
Pseudorhytidopilus detonii |
Certosa di Vedana |
Shells of different sizes.[18] |
A Marine Gasteropod (Limpet) of the Family Acmaeidae inside Patellogastropoda. |
|
|
Proacirsa[18] |
Proacirsa (Schafbergia) crenata |
Certosa di Vedana |
Shells of different sizes.[18] |
A Marine Gasteropod (Snail) of the Family Gordenellidae inside Allogastropoda. |
|
|
Discohelix excavata |
Certosa di Vedana |
Shells of different sizes.[18] |
A Marine Gasteropod (Snail), type genus of the Family Discohelicidae inside Vetigastropoda. |
| |
|
Eucyclidae Indeterminate |
Certosa di Vedana |
Shells of different sizes.[18] |
A Marine Gasteropod (Snail) of the Family Eucyclidae inside Seguenzioidea. |
||
|
Eucyclus[18] |
Eucyclus (Lokuticyclus) kericserensis |
Certosa di Vedana |
Shells of different sizes.[18] |
A Marine Gasteropod (Snail), type genus of the Family Eucyclidae inside Seguenzioidea. |
|
|
Austriacopsis[18] |
Austriacopsis austriaca |
Certosa di Vedana |
Shells of different sizes.[18] |
A Marine Gasteropod (Snail) of the Family Fissurellidae inside Fissurelloidea. |
|
|
Emarginula (Emarginula) vadanaei |
Certosa di Vedana |
Shells of different sizes.[18] |
A Marine Gasteropod (Snail) of the Family Fissurellidae inside Fissurelloidea. |
| |
|
Anticonulus[18] |
Anticonulus acutus |
Certosa di Vedana |
Shells of different sizes.[18] |
A Marine Gasteropod (Top Snail) of the Family Trochidae inside Trochoidea. |
|
|
Plectotrochus[18] |
Plectotrochus sp. |
Certosa di Vedana |
Shells of different sizes.[18] |
A Marine Gasteropod (Top Snail) of the Family Trochidae inside Trochoidea. |
|
|
Ataphrus[18] |
*Ataphrus (Ataphrus) latilabrus *Ataphrus (Ataphrus) cordevolensis |
Certosa di Vedana |
Shells of different sizes.[18] |
A Marine Gasteropod (Snail), type genus of the Family Ataphridae inside Trochoidea. |
|
Thylacocephala
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
Rugocaris[19] |
|
|
Medium-sized bivalved carapace |
A Concavicaridan Thylacocephalan. This specimen is a rathin rare case where there was the discover of a Thylacocephalan specimen in a rock deposed in a well oxigenated environment, while other finds come mostly from were from anaerobic environments.[19] Rugocaris lived in an epibathyal environment.[19] |
|
Crustacea
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
Pustulina[12] |
Pustulina sp. |
Valbona Area |
Chelae |
An Erymidae Decapodan. There is a frequent presence of Thalassinoides burrows associated with Pustulina body fossils.[12] |
 Pustulina |
|
Phlyctisoma[12] |
Phlyctisoma sinemuriana |
Valbona Area.[12] |
Slightly deformed Exuvia |
An Erymid Decapodan Crustacean common on In mediterranean rocks. With a rostrum about 1.3 cm long and the cephalic part of carapace about 2.5 cm the specimen probably reached a total length between 9 and 10n cm, being one of the largest specimens belonging to this genus. Frequent association with Thalassinoides burrows. A complete seafloor section was fossilized.[12][11] |
|
|
Phraterfabanella[20] |
Phraterfabanella tridentinensis |
Tonezza del Cimone.[20] |
Valves |
An Ostracodan of the family Cytherideidae inside Cytheracea. The assemblage is dominated (>95%) by this taxon.[20] It is a rather Medium-sized Ostracodan and markedly sexually dimorphic (males more elongate and more subrectangular versus shorter, more inflated and more subtriangular females).[20] it is likely that the palaeoenvironment was somewhat "stressed" and probably influenced by Salinity, where this genus would adapt better that Other Ostracodans (is related to the modern euryhaline species, Cyprideis torosa).[20] |
|
|
Klieana[20] |
Klieana sp. |
Tonezza del Cimone.[20] |
Valves |
An Ostracodan of the family Cytherideidae inside Cytheracea. The earliest record of the genus, the next youngest records of the genus are from Middle Jurassic sequences of France and Great Britain.[20] |
|
|
Limnocythere sp. |
Tonezza del Cimone.[20] |
Valves |
An Ostracodan of the family Limnocytherinae inside Cytheracea. High probability to be a new species of Limnocythere since the authors know of no other with similar posterolateral sulcation.[20] |
||
Vertebrata
Chondrichthyes
Episodic surficial bioturbation is common on the Rotzo Formation, due to invertebrates or fishes which alter intensely but rapidly the substrate for many cm in depth.[10] It this case the Bioturbation is assigned to mollusc predatory Chondrichthyes, such as Hybodontidae and Heterodontidae.[10] It also resembles present day flat angel sharks or Squatinidae and Guitarfish such as Rhinobatos.[10]
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
Orthacodus sp |
|
Teeth |
A Shark, type genus of the family Orthacodontidae inside Synechodontiformes. The teeth recovered resemble Orthacodus longidens, and are related to an epibathyal environment, near to a major carbonate platform shelf. |
||
|
Hybodus sp. |
|
First dorsal fin spine |
A Shark, type genus of the family Hybodontidae inside Hybodontiformes. A very prolific genus, found mostly on open marine units. |
||
Actinopterygii
Unidentified fish scales are known from the formation.[22]
| Genus | Species | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|
|
Semionotiformes Indeterminate |
Campiluzzi Tunnel, west of Monte Buso. |
The assigned teeth where found on a layer referred to a Carbonate Platform nearshore section, probably a Lagoonar Environment, where fhis and marine crocodrylomorphs live. |
| ||
|
Pycnodontiformes indeterminate |
Campiluzzi Tunnel, west of Monte Buso. |
|
Teleostei Fishes of small size, related to lagoonar environments |
||
|
Pholidophoriformes Indeterminate |
Campiluzzi Tunnel, west of Monte Buso. |
|
Teleostei fishes, with genera know to form large Fish schools. |
||
Crocodyliformes
| Genus | Species | Location | Material | Notes | Images |
|---|---|---|---|---|---|
|
Teleosauridae? Indeterminate |
Monte Pasubio |
A Thalattosuchian Mesoeucrocodylian. It was cited the presence of fragmentary and poorly preserved remains of “Teleosauridae?”. There are at least two morphotypes, implying two genera or two species. The fossils were found on lagoonal deposits.[22] |
 Example of Thallatosuchian, Macrospondylus | ||
|
aff. Eopneumatosuchus sp. |
Monte Pasubio |
Partial skull and teeth.[23] |
A basal crocodyliform. Was originally thought to be Thalattosuchian remains. Has been compared recently with Eupneumatosuchus, is a member of the genus or a closely related species. |
||
|
Metasuchia Indeterminate |
Monte Pasubio |
Upper jaw with rounded teeth.[23] |
A basal crocodyliform. It has rouded teeth that suggest a Duriphagous Diet. |
||
Dinosaurs
On the Inter-supratidal levels show that on the Rotzo Formation the Tracksites were rarely hit by Storm Waves.[25] Bella Lastra Tracksite recovers this environment, where the shales present (Where Fish & Crocodrylomorph Remains where found) are filled with plant roots, pollen grains, spores, freshwater ostracodes and the bivalve Eomiodon.[25] This was deposited mostly on a Lagoonar environment with abundant shed vegetation.[25] The main local Track record recovers specially Theropoda and Sauropoda, where the Sauropods are the most abundant tracks present (70%), moving the Otozum-like Sauropodomorphs of lower levels, with the climate changing from arid to humid.[25]
Color key
|
Notes Uncertain or tentative taxa are in small text; |
| Dinosaurs of the Rotzo Formation | ||||||
|---|---|---|---|---|---|---|
| Genus | Species | Location | Member | Material | Notes | Images |
|
Moyenisauropus sp. |
|
Footprints |
Is considered synonymous with the ichnogenus Anomoepus. The tracks adscribed share some morphological affinity with those referred to the Ankylosauridae, such as the ichnogenera Metatetrapodus and Tetrapodosaurus, and probably belonged to medium-sized Scelidosaurs or other kind of Thyreophorans. Include Specimens of up to 30 cm, suggesting +4 m long scelidosauroids. |
|||
|
|
Footprints |
Probably related to Coelophysidae, such as Procompsognathus and Panguraptor or Coelophysoidea, such as Lophostropheus. All tracks were probably produced by individuals with the same functional anatomy of the hind foot.[25] |
|||
|
|
Footprints |
Includes Kayentapus sp. assigned to Sinosaurus-alike Theropods, but on the Rotzo Formation include also Abelisauroid-like tracks, similar to the foot of the genus Velocisaurus.[22] The tracks measure 30 cm long and have a distinctive robust digit III.[25] |
|||
|
Parabrontopodus sp. A Parabrontopodus sp. B |
|
Footprints |
Tracks from large basal members of Sauropoda. The larger tracks comprise elliptic pes (L=70 cm; W=50 cm) and subcirluar manus prints (L=33 cm; W=30 cm), what are among the largest known dinosaur tracks of the lower jurassic.[25] While nearly destroyed, the Tracks resemble the foot of the genus Gongxianosaurus, but also Barapasaurus. There is a type B of Parabrontopodus slightly smaller that resemble the genus Vulcanodon. |
|||
Flora
There are abundant fossils of freshwater algae, like Botryococcus and Pseudoschizaea.[30]
Amber
| Genus | Species | Location | Stratigraphic position | Material | Notes |
|---|---|---|---|---|---|
|
Non Assignable Species |
Bellori village |
Amber Fragments |
The Lessini Mountains Amber represents the first report of Jurassic amber from Italy and one of the very few in the world. Due to being composed by drops of less than 1 mm with preserved exceptionally intact morphologies the Bellori amber was probably neither environmentally stressed nor affected by diseases.[31] While no animal remains were found inside the Bellori Amber so far there are various traces of very small (< 1 mm) vegetal fragments, here identified as tissue remains of wood and “mummified wood”.[31] It has also a large amount of Circumpolles (Cheirolepidiaceae), and in some fragments there are traces of the freshwater algae Pseudoschizaea.[31] Although several cuticles found in Bellori could be attributed to Pagiophyllum (Araucariaceae).[31] Those lived on a coastal and wet palaeoenvironment similar to the present-day Taxodium swamps with monsoonal seasons as in the modern southern Asia.[31] | ||
Palynology
| Genus | Species | Location | Stratigraphic position | Material | Notes |
|---|---|---|---|---|---|
|
Accincitisporites sp. |
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Unknown affinities | ||
|
Alisporites[32] |
Alisporites sp. |
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Voltzia (Willsiostrobus), Corystospermales, Ginkgoopsida (Pelataspermales), Coniferopsida (Podocarpaceae, Ulmanniaceae, Voltziales).[31] | |
|
Aratrisporites[32] |
Aratrisporites sp |
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Lycophytes, in situ in Cyclostrobus, Lycostrobus and Annalepis zeiller.[31] | |
|
Auritulinasporites[32] |
Auritulinasporites scanicus |
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Pteridophyta.[31] | |
|
Baculatisporites[32] |
Baculatisporites sp |
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Pteridopsida.[31] | |
|
Calamospora[32] |
Calamospora sp |
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Sphenopsida.[31] | |
|
Camarozonosporites[32] |
Calamospora sp |
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Lycopsida.[31] | |
|
Cabochonicus[32] |
cf. Cabochonicus carbunculus |
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[33] |
Affinities with Selaginellaceae | |
|
Chasmatosporites[32] |
Chasmatosporites sp |
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Lycopsida.[31] | |
|
Classopollis[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Pollen.[31] |
Affinities with Cheirolepidiaceae.[31] | |
|
Concavisporites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Pteridophyta.[31] | |
|
Cycadopites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Cycadophyta.[31] | |
|
Deltoidospora[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Lycophyta.[31] | |
|
Densosporites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Lycophyta.[31] | |
|
Eucommidites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
||
|
Foveosporites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Selaginellaceae.[31] | |
|
Granuloperculatipollis[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Pollen.[31] |
Affinities with Selaginellaceae.[31] | |
|
Horstisporites[32] |
Horstisporites harrisii |
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[33] |
Affinities with Selaginella-like | |
|
Hughesisporites[32] |
cf. Hughesisporites orlowskae |
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[33] |
Affinities with Lycophyta | |
|
Ischyosporites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Pteridopsida.[31] | |
|
Leptolepidites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Lycopsida.[31] | |
|
Limbosporites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Lycopsida.[31] | |
|
Lycopodiacidites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Lycopsida.[31] | |
|
Lycopodiumsporites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Lycopsida.[31] | |
|
Monosulcites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Pteridopsida.[31] | |
|
Perinopollenites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Pollen.[31] |
Affinities with Gymnospermophyta.[31] | |
|
Pinuspollenites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Pollen.[31] |
||
|
Retitriletes[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Lycopodiaceae.[31] | |
|
Retusotriletes[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Lycopodiaceae.[31] | |
|
Skarbysporites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
||
|
Schizosporis[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Chlorophyta.[31] | |
|
Spheripollenites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Pollen.[31] |
Affinities with Chlorophyta.[31] | |
|
Tigrisporites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
||
|
Todisporites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Pteridopsida.[31] | |
|
Trachysporites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Pteridopsida.[31] | |
|
Trileites[32] |
cf. Trileites murrayi |
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[33] |
Affinities with Selaginellaceae | |
|
Verrutriletes[32] |
cf. Verrutriletes compostipunctatus |
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[33] |
Affinities with Selaginellaceae | |
|
Vitreisporites[32] |
|
Bellori, Ponte Basaginocchi, Vajo dell’Anguilla |
Spores.[31] |
Affinities with Gymnospermophyta.[31] | |
Plant remains
| Genus | Species | Location | Stratigraphic position | Material | Notes | Images |
|---|---|---|---|---|---|---|
|
|
Stems |
Affinities with Equisetaceae inside Polypodiopsida. Related to humid environments, the stems of local Equisetopsids show a rather large grown cycle, like the Bamboo on the modern Southern Asia, implicating tall Plants influenced by a Tropical Climate. |
|||
|
|
Leaf Whorl |
Affinities with Phyllothecaceae inside Equisetales |
|||
|
|
Fronds |
Affinities with Dipteridaceae inside Gleicheniales. A rather lower Fern, with great resemblance with the modern genus Dipteris |
|||
|
|
Fronds |
Affinities with Matoniaceae inside Gleicheniales. |
|||
|
|
Fronds |
Affinities with Matoniaceae inside Gleicheniales. |
|||
|
|
Fronds |
Affinities with Matoniaceae inside Gleicheniales. |
|||
|
|
Fronds |
Affinities with Dipteridaceae inside Gleicheniales. |
|||
|
|
Fronds |
Affinities with Polypodiales inside Polypodiopsida |
|||
|
|
Fronds |
Affinities with Lyginopteridopsida inside Lyginopteridales |
|||
|
|
Fronds |
Affinities with Cyclopteridaceae inside Pteridospermatophyta. |
|||
|
|
Fronds |
Affinities with Corystospermales inside Pteridospermopsida. On the Roverè di Velo collection, C. brauniana is the most common Frond found. The Fronds belong to medium to large Arboreal Ferns. |
 Cycadopteris brauniana and Cycadopteris sp., both recovered from different locations of the Rotzo Formation | ||
|
|
Fronds |
Affinities with Corystospermales inside Pteridospermopsida. This frons genus has been Synonymized with Pachypteris , but it clearli differs due to the presence of odontopteridian pinnules, while Pachypteris has pinnules of the sphenopteridian type. Related to Arboreal Ferns. |
 Dichopteris visianica from the Rotzo Formation | ||
|
|
Leaflets |
Affinities with Caytoniales inside Pteridospermopsida. |
|||
|
|
Leaflets |
Affinities with Caytoniaceae inside Caytoniales. There is a superficial doubt with the assigantion to S. goeppertiana, and du to that Roverè di Velo specimen may be confirmed by comparing them with original Zigno's Material. |
|||
|
Grey limestones of Veneto |
Leaflets |
Affinities with Bennettitales inside Cycadeoidopsida. Related with Cycad-like trees. |
|||
|
Grey limestones of Veneto. |
Reproductive structure |
Affinities with Bennettitales inside Cycadeoidopsida. Weltrichia is considered by some authors some kind of Bennetitalean Flower, putting that group on relationships with the Angiosperms. |
|||
|
Grey limestones of Veneto. |
Leaflets |
Affinities with Bennettitales inside Cycadeoidopsida. This genus has been related with the more arboreal family Williamsoniaceae, although is more probably from a low arboreal to arbustive Bennetite. |
|||
|
|
Pinnate leaf fragments |
Affinities with Bennettitales inside Cycadeoidopsida. Overall, the genus Otozamites is among the most abundant flora genus recovered on some of the levels of the Rotzo Formation, and also one of the most diversified. It belongs to arbustive Bennetites. |
 Otozamites bunburyanus from the Rotzo Formation | ||
|
|
Leaves |
Affinities with Bennettitales. Was previously ascribed by Guiscardi (Director of the Geology Department of the Napoles University between 1861 al 1885) to Pachypteris visianica and Cycadopteris brauniana. |
 Ptilophyllum grandifolium from the Rotzo Formation | ||
|
|
Leaves |
Affinities with the genus Trichopitys, as probably a member of Ginkgoales inside Ginkgoopsida. |
|||
|
Roverè di Velo |
Incomplete leaves |
Affinities with Ginkgoaceae inside Ginkgoales. Was assigned the Podozamites genus and named them Podozamites zeillerianus. |
|||
|
Grey limestones of Veneto. |
Branched shoots |
Affinities with Palyssia-like, a ganus that has been related to the fossil wood Phyllocladoxylon, being probably Fronds of the Podocarpaceae family. |
|||
|
|
Branched shoots |
Affinities with Pinaceae inside Pinales. This genus resembles the modern Abies, with similar distribution of the leaves. |
|||
|
Grey limestones of Veneto. |
Branched shoots |
Affinities with Podocarpaceae inside Coniferales. |
|||
|
|
Branched shoots |
Affinities with Araucariaceae inside Coniferales. Brachyphyllum tropidimorphyrn shows close resemblance between African and Venetian conifers and its distribution suggests a lowland araucarian forest.[39] |
|||
|
Roverè di Velo |
Leaves |
Affinities with Araucariaceae inside Coniferales. One of the specimens was assigned to Otozamites massalongianus, due to confusing the overlapping appearance and the Otozamites-like shape of the leaves of the apical portion of the main shoot. |
 Pagiophyllum rotzoanum from the Rotzo Formation | ||
|
Roverè di Velo |
Nearly complete pinna |
Due to the bad preservation of specimen no systematic attribution is possible. |
|||
See also
- List of dinosaur-bearing rock formations
Bibliography
- https://paleobiodb.org/classic/basicCollectionSearch?collection_no=88592
- Broglio Loriga, C., & Neri, C. (1976). Aspetti paleobiologici e paleogeografici delle facies “Lithiotis” (Giurese inf.) Rivista Italiana di Paleontologia e Stratigrafia, 82, 651–151.
- Franceschi, M., Dal Corso, J., Posenato, R., Roghi, G., Masetti, D., & Jenkyns, H. C. (2014). Early Pliensbachian (Early Jurassic) C-isotope perturbation and the diffusion of the Lithiotis Fauna: Insights from the western Tethys. Palaeogeography, Palaeoclimatology, Palaeoecology, 410, 255–263. doi:10.1016/j.palaeo.2014.05.025
- P. Mietto, G. Roghi, and R. Zorzin. 2000. Le impronte di dinosauri liassici dei Monti Lessini Veronesi [The Liassic dinosaur tracks from the Veronese Monti Lessini]. Bollettino del Museo Civico di Storia Naturale di Verona. Geologia Paleontologia Preistoria 24:55-72
- MASETTI D; POSENATO R; BASSI D.; FUGAGNOLI A (2005) The Rotzo Formation (Lower Jurassic) at the Valbona Pass (Vicenza Province). STAMPA
- Cyclical variation in paleoenvironments of the Rotzo formation (Lower Jurassic, Lessini Mts., N Italy) / Neri, Mirco; Papazzoni, Cesare Andrea; Vescogni, Alessandro; Roghi, Guido. - STAMPA. - (2015), pp. 74-75. ((Intervento presentato al convegno Tenth Romanian Symposium on Paleontology tenutosi a Cluj-Napoca, Romania nel 16–17 October 2015.
- Urban, I. (2017). Petrografia e geochimica delle ooliti del Giurassico inferiore della Piattaforma di Trento.
- Monaco, P., & Giannetti, A. (2001). Stratigrafia tafonomica nel Giurassico inferiore dei Calcari Grigi della Piattaforma di Trento. Atti Ticinensi di Scienze della Terra, 42, 175-209.
- R. Posenato. 2013. Opisoma excavatum Boehm, a Lower Jurassic photosymbiotic alatoform-chambered bivalve. Lethaia 46:424-437
- Monaco, P., & Giannetti, A. (2002). Three-dimensional burrow systems and taphofacies in shallowing-upward parasequences, lower Jurassic carbonate platform (Calcari Grigi, Southern Alps, Italy). Facies, 47(1), 57-82.
- MONACO P. (2000). Decapod burrows (Thalassinoides, Ophiomorpha) and crustacean remains in the Calcari Grigi, lower Jurassic, Trento platform (Italy). 1st Workshop on Mesozoic and Tertiary decapod crustaceans, Studi e Ricerche, Associazione Amici del Museo Civico “G.Zannato” Montecchio Maggiore (Vicenza), October 6–8, 2000, pp.55-57
- A. Garassino and M. Monaco. 2000. Burrows and body fossil of decapod custaceans i the Calcari Grigi, Lower Jurassic, Treno Platform (Italy)
- MONACO P. & GARASSINO A. (2001). Burrowing and carapace remains of crustacean decapods in the Calcari Grigi, Early Jurassic, Trento platform. Geobios, 34 (3), 291-301.
- Giannetti, A., Monaco, P., Caracuel Martín, J. E., Soria Mingorance, J. M., & Yébenes Simón, A. (2007). Functional morphology and ethology of decapod crustaceans gathered by Thalassinoides branched burrows in Mesozoic shallow water environments.
- P. Mietto. 1985. Ammoniti nella Piattaforma liassica Veneta. Rivista Italiana di Paleontologia e Stratigrafia 91(1):3-14
- HAAS, O., 191 3 - Die Fauna des mittleren Lias von Ballino im Sudtirol: Beitr. Paldont. Geol. Osterr.-Ungam u Orient, 26, 1-161. MCNAMARA, K.J. , 198 6 - A guide to the nomenclature of heterochrony: Journal of Pal., 60 (1), 4-13
- CASTELLI, M. (1980). Ammoniti del Pliensbachiano della collezione paleontologica del Museo civico di storia naturale di Brescia. Natural Disaster Reduction in China, (1), 2.
- R. Gatto and S. Monari. 2010. Pliensbachian gastropods from Venetian Southern Alps (Italy) and their palaeobiogeographical significance. Palaeontology 53(4):771-802
- Tintori, A., Bigi, E., Crugnola, G., & Danini, G. (1986). A new Jurassic Thylacocephala Rugocaris indunensis gen. n. sp. n. and its paleoecological significance. Rivista Italiana di Paleontologia e Stratigrafia, 92(2), 239-250.
- Boomer, I., Whatley, R., Bassi, D., Fugagnoli, A., & Loriga, C. (2001). An Early Jurassic oligohaline ostracod assemblage within the marine carbonate platform sequence of the Venetian Prealps, NE Italy. Palaeogeography, Palaeoclimatology, Palaeoecology, 166(3-4), 331-344.
- Franceschi & Bernardi (2020). Vertebrate remains from the Rotzo Formation (Lower Jurassic, Trento Platform, Italy): preliminary note. Fossilia, Volume 2020: 25-27. https://doi.org/10.32774/FosRepPal.2020.0607 Fossilia - Reports in Palaeontology
- Petti, F. M., Bernardi, M., Todesco, R., & Avanzini, M. (2011). Dinosaur footprints as ultimate evidence for a terrestrial environment in the late Sinemurian trento carbonate platform. Palaios, 26(10), 601-606.
- Avanzini, M., 1998. Resti di vertebrati dal Giurassico inferiore della piattafor-ma di Trento (Italia settentrionale) Nota prelimiare. Studi Trentini diScienze Naturali. Acta Geologica 73 (1996)
- Avanzini M., 1998 – Resti di rettili continentali dal Giurassico inferiore della piattaforma di Trento (Italia settentrionale). Studi Trentini di Scienze Naturali - Acta Geologica, Trento, 73: 75-80
- GUIDOROGHI, R. (2006). LOWER JURASSIC (HETTANGIAN–SINEMURIAN) DINOSAUR TRACK MEGASITES, SOUTHERN ALPS, NORTHERN ITALY. The Triassic-Jurassic Terrestrial Transition: 37, 37, 207.
- M. Avanzini, G. Leonardi, R. Tomasoni and M. Campolongo. 2001. Enigmatic dinosaur trackways from the Lower Jurassic (Pliensbachian) of the Sarca Valley, northeast Italy. Ichnos 8:235-242
- Petti F.M., Avanzini M., Antonelli M., Bernardi M., Leonardi G., Manni R., Mietto P., Pignatti J., Piubelli D., Sacco E., Wagensommer A. 2020 (in press). Jurassic tetrapod tracks from Italy: a training ground for generations of researchers. In: Romano M., Citton P. (Eds.), Tetrapod ichnology in Italy: the state of the art. Journal of Mediterranean Earth Sciences 12, xx-xx.
- M. Avanzini and F. M. Petti. 2008. Updating the dinosaur tracksites from the Lower Jurassic Calcari Grigi Group (Southern Alps, northern Italy). Studi Trentini di Scienze Naturali, Acta Geologica 83:289-301
- Franceschi M., Martinelli M., Gislimberti L., Rizzi A., Massironi M. (2015) - Integration of 3D modeling, aerial LiDAR and photogrammetry to study a synsedimentary structure in the Early Jurassic Calcari Grigi (Southern Alps, Italy). European Journal of Remote Sensing, 48: . doi:
- Neri, M., Kustatscher, E., Roghi, G., & Papazzoni, C. A. (2016). Paleobotanical assemblage from the Lower Jurassic amber bearing levels from the Rotzo Formation, Monti Lessini (Venetian Prealps, Northern Italy). In The Micropaleontological Society, 5th Silicofossil and Palynology Joint Meeting (pp. 33-33). ITA.
- Neri, M., Roghi, G., Ragazzi, E. & Papazzoni, C. A. 2017. First record of Pliensbachian (Lower Jurassic) amber and associated palynoflora from the Monti Lessini (northern Italy). Geobios 50, 49–63.
- Van Erve, A.W., 1977. Palynological investigation in the Lower Jurassic of the Vicentinian Alps (Northeastern Italy). Review of Palaeobotany and Palynology 23, 1-117
- Neri, M., Kustatscher, E., & Roghi, G. (2018). Megaspores from the Lower Jurassic (Pliensbachian) Rotzo Formation (Monti Lessini, northern Italy) and their paleoenvironmental implications. In S. Slater, E. Kustatscher & V. Vajda, (Eds.), Jurassic biodiversity and terrestrial environments. Palaeobiodiversity and Palaeoenvironments, 98(1)
- De Zigno, A., 1856–1885. Flora fossilis formationis Oolithicae. Tipografia del Seminario di Padova, 1–2, 426pp.
- De Zigno, A., 1885. Flora fossilis formationis oolithicae. Vol. 2. Padova, Tip. Del Seminario.
- A. Wesley (1956) Contributions to the knowledge of the flora of the Grey Limestones of Veneto, Part 1 Mem. Ist. Geol. Min. Univ. Padova, 19 , pp. 1-69
- A. Wesley (1958) Contributions to the knowledge of the flora of the Grey Limestones of Veneto, Part 2 Mem. Ist. Geol. Min. Univ. Padova, 21, pp. 1-57
- Bartiromo A. & Barone Lumaga M.R. (2009). Taxonomical revision of the Collection of Jurassic plants from Roverè di Velo (Veneto, northern Italy) stored in the Palaeontological Museum of the University of Naples “Federico II”. Bollettino della Società Paleontologica Italiana, 48: 1-13A.
- Krassilov, V. A. (1978). Araucariaceae as indicators of climate and paleolatitudes. Review of Palaeobotany and Palynology, 26(1-4), 113-124.


.jpg.webp)
