Smooth newt
The smooth newt, northern smooth newt or common newt (Lissotriton vulgaris) is a species of newt. It is widespread in much of Eurasia, from the British Isles to Siberia and northern Kazakhstan, and introduced to Australia. Individuals are brown with an orange to white, spotted underside and reach a length of 8–11 cm (3.1–4.3 in), with males being larger than females. The skin is dry and velvety while the newts live on land but becomes smooth when they migrate into water for breeding. Breeding males develop a more vivid colour pattern and a conspicuous skin seam (crest) on their back.
| Smooth newt | |
|---|---|
 | |
| Male during land phase | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Amphibia |
| Order: | Urodela |
| Family: | Salamandridae |
| Genus: | Lissotriton |
| Species: | L. vulgaris |
| Binomial name | |
| Lissotriton vulgaris (Linnaeus, 1758) | |
| Subspecies[2] | |
 | |
| Synonyms | |
|
48,[3] including: | |
Originally described by Carl Linnaeus as a lizard, the smooth newt went by different genus names before the current classification in Lissotriton was adopted. Three subspecies are currently accepted. Four former subspecies, all with more restricted ranges, are now classified as separate species, as they are distinct in appearance and genetically: the Caucasian, the Greek, Kosswig's and Schmidtler's smooth newt. The smooth newt forms a species complex with these four species and the Carpathian newt and hybridises with some of them.
Smooth newts live on land for most of the year, where they are mostly nocturnal and hide during the day. They can adapt to a wide range of natural or semi-natural habitats, from forests over field edges to parks and gardens. The newts feed mainly on various invertebrates such as insects or earthworms and are themselves eaten by predators such as fish, birds or snakes. Between spring and summer, they breed in ponds or similar water bodies. Males court females with a ritualised underwater display. Females then lay their eggs on water plants, and larvae hatch after 10 to 20 days. They develop over around three months before metamorphosing into terrestrial juveniles (efts). Maturity is reached after two to three years, and adults can reach an age of up to 14 years.
The smooth newt is common over much of its range and classified as Least Concern species by the IUCN. It is however negatively affected by habitat destruction and fragmentation and the introduction of fish. Like other European amphibians, it is listed in the Berne Convention as a protected species.
Taxonomy
Swedish naturalist Carl Linnaeus described the smooth newt in 1758 as Lacerta vulgaris, placing it in the same genus as the green lizards.[4]:370 It was later re-described under several different species and genus names, including Triton, Molge, Salamandra and Lissotriton, with in total 48 species synonyms published.[3] Most recently, it was included in the genus Triturus, along with most European newts.[5]:221 This genus however was found to be polyphyletic, containing several unrelated lineages,[6][7][8] and the small-bodied newts, including the smooth newt, were therefore split off as separate genus in 2004 by García-París and colleagues.[9]:233 They used the name Lissotriton, introduced by the English zoologist Thomas Bell in 1839 with the smooth newt as type species[10]:132 but then considered a synonym of Triturus.[3] "Lissotriton" is a combination of Greek λισσός, lissós for "smooth" and the name of Triton, an ancient Greek god of the sea, while the species epithet vulgaris means "common" in Latin.[11]:17
Three subspecies are accepted by Pabijan, Wielstra and colleagues: L. v. vulgaris, L. v. ampelensis and L. v. meridionalis.[2][12] These authors, followed by Amphibian Species of the World,[3] recognise four former subspecies from southern Europe and west Asia as separate species, as they are morphologically and genetically distinct: the Greek smooth newt (L. graecus), Kosswig's smooth newt (L. kosswigi), the Caucasian smooth newt (L. lantzi) and Schmidtler's smooth newt (L. schmidtleri). The five smooth newt species and the Carpathian newt (L. montadoni), which is their sister species, have collectively been referred to as the "smooth newt species complex".[12]
To distinguish the smooth newt from its close relatives, the English name "northern smooth newt" has been suggested.[12] Other common names that have been used in the literature include: common newt, great water-newt, common water-newt, warty eft, water eft, common smooth newt, small newt, small eft, small evet, and brown eft.[3]
Evolution
Molecular phylogenetic analyses have shown that the smooth newt is distinct from its four close relatives – the Caucasian, Greek, Kosswig's, and Schmidtler's smooth newt – which were formerly considered subspecies (see section Taxonomy above). The relationships within this species complex have however not been fully resolved. Within the smooth newt itself, genetic groups do not completely match the currently accepted subspecies (ampelensis, meridionalis, vulgaris), described based on morphology.[2] The five smooth newt species collectively were estimated to have diverged from the Carpathian newt around four to six million years ago.[13][14]
Genetic analyses have also demonstrated ongoing gene flow between the smooth newt and its relatives. Although the Carpathian newt is morphologically clearly different, hybridisation between the two species is frequent;[11]:26 it has been shown that smooth newt mitochondrial DNA has introgressed into and completely replaced that of the Carpathian newt populations.[15] Partial introgression also occurred from the smooth newt to the Greek smooth newt.[2] These patterns are likely due to the range expansion and secondary contact of species after the Last Glacial Maximum, which they likely survived in refugia mainly in southern and eastern Europe.[15][14][16] The palmate newt (Lissotriton helveticus), although often occurring in the same habitats, almost never hybridises with the smooth newt.[11]:25 Artificial crosses with even more distant species such as the alpine (Ichthyosaura alpestris) and northern crested (Triturus cristatus) newts were however successful in laboratory experiments.[11]:29
Description
_cropped.jpg.webp)



General characteristics
Adult males of the smooth newt reach around 9–11 cm (3.5–4.3 in) head-to-tail length and are thus slightly larger than the females, which reach 8–9.5 cm (3.1–3.7 in). The body weight of adults varies between 0.3 and 5.2 g, and decreases during the breeding season. The head is longer than it is wide, with 2–3 longitudinal grooves on the top, and the elongated snout is blunt in the male and rounded in the female. The skin is velvety and water-repellent on land but smooth during the aquatic phase; it contains mucus and toxin glands and its upper layer is shed off regularly.[11]:80–93[5]:233–234
Outside the breeding season, both sexes are yellow-brown, brown or olive-brown. The male has dark, round spots, while the female has smaller spots of the same colour, which sometimes form two or more irregular lines along the back. The male has an orange strip on the tail underside, and the throat and belly in males are orange to white with small dark, rounded spots (these are lighter with smaller spots in the female). Size and colour vary with environment, and the newts tend to be smaller in northern latitudes.[11]:80–93[5]:233–234 Albinistic and leucistic individuals have been described.[11]:94[17]
The smooth newt is diploid (i.e. it has two copies of each chromosome), with 24 chromosomes in total.[11]:107
Breeding characteristics
During the aquatic breeding season, males develop a skin seam or crest, which runs uninterrupted along the back and the tail. It is 1–1.5 mm high at mid-body, but higher along the tail. The tail also has a lower fin, and its end is pointed. The cloaca (the single digestive, urinary and reproductive orifice) of breeding males is swollen, round and dark-coloured. The hindfeet have more or less developed toe flaps, depending on the subspecies. Colours in general are more vivid than during the land phase. The dark spots grow larger, and the crest often has vertical dark and bright bands. There are five to seven longitudinal stripes on the head. The lower edge of the tail is red with a silver-blue flash and black spots. Females only develop low, straight tail fins but no crest or toe flaps, and are more drably coloured.[18]:26[5]:233–234
Subspecies differ slightly in male secondary characteristics: L. v. ampelensis has strongly developed toe flaps, its tail tapers into a fine thread (but not a distinct filament), and the body is slightly square in cross-section. L. v. meridionalis also has toe flaps and a pointed tail, its crest is smooth-edged, and its body is square-shaped. In the nominate subspecies, L. v. vulgaris, the crest is clearly denticulated, toe flaps are only weakly developed and the body is round.[5]:234–236
Larvae
The aquatic larvae are 6.5–7 mm long and yellow-brown with two longitudinal stripes at hatching. They initially have, in addition to their gills, only two balancers at the sides of the head, short appendages for attaching to plants which get resorbed within a few days.[5]:237 As in all salamanders, forelegs develop before the hindlegs. The colour becomes a more cryptic, darkly marbled yellow to brown in the growing larvae. Larvae are very slender and similar to the palmate newt. They develop a skin seam from the neck to the pointed tail; the tail is as long as the head and trunk. The larvae grow to 3–4.5 cm (1.2–1.8 in), which is also the size of the efts (the terrestrial juveniles) just after metamorphosis.[11]:188–192
Similar species
The smooth newt resembles the other, less widespread Lissotriton species. It can be confused especially with the closely related "smooth newt complex" species (marked with * in the table below) and the more distant palmate newt, which often occurs in the same area.[12][11]:25 Females are especially difficult to tell apart, as distinguishing features are mainly observed in the males at breeding season.[11]:19–41[5]:225–235
| Species | Distribution | Breeding male characteristics | Other | |||
|---|---|---|---|---|---|---|
| Body shape | Dorsal crest | Toe flaps (hind feet) | Tail end | |||
| Smooth newt* L. vulgaris | widespread from British Isles to Central Asia | round to square (depending on subspecies) | smooth or denticulated (depending on subspecies) | weakly to well developed (depending on subspecies) | pointed to elongated, no filament | |
| Bosca's newt L. boscai | West Iberian peninsula | slightly square | none | none | short filament | belly with some dark spots, especially at sides |
| Carpathian newt* L. montandoni | Carpathians | square | very low, smooth-edged | weakly developed | blunt, with filament | belly unspotted |
| Caucasian smooth newt* L. lantzi | Caucasus | slightly square | high (>1 mm at mid-body), denticulated (almost spine-shaped) | moderately developed | pointed, but no filament | |
| Greek smooth newt* L. graecus | Southern Balkans | square | low (<1 mm at mid-body), smooth-edged | well developed | long filament | lower tail fin unspotted |
| Italian newt L. italicus | Southern Italy | slightly square | none | none | pointed, no filament | very small, 4.5–7.5 cm (1.8–3.0 in); throat with few or no spots; golden-yellow patch behind eyes in both sexes |
| Kosswig's smooth newt* L. kosswigi | Northern Anatolia | square | low (<1 mm at mid-body) but higher at tail base | strongly developed | long filament | |
| Palmate newt L. helveticus | Western Europe | square | low, smooth-edged | strongly developed | long filament (both sexes) | throat unspotted |
| Schmidtler's smooth newt* L. schmidtleri | Anatolia and eastern Balkans | slightly square | high (>2 mm at mid-body), denticulated | weakly developed | elongated, no filament | very small, 5–7 cm (2.0–2.8 in) |
Distribution
Native range
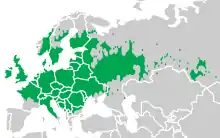
The smooth newt has been described as "the most ubiquitous and widely distributed newt of the Old World".[5]:237 The nominate subspecies, L. v. vulgaris, is most widespread and ranges natively from Ireland (where the smooth newt is the only newt species[11]:42) and Great Britain in the west to Siberia and northern Kazakhstan in the east. In the north it reaches central Fennoscandia, and its southern limit is central France, northern Italy, the central Balkans and the dry Eurasian steppe of Ukraine and Russia.[19][12][5]:234–238[11]:42–44 The subspecies L. v. ampelensis only occurs in the Carpathians of Ukraine and the Danube delta of northern Romania, and L. v. meridionalis in the northern half of Italy, southern Switzerland, Slovenia and Croatia.[5]:234–235
In the Carpathians, the smooth newt generally prefers lower elevations than the Carpathian newt. In the Balkans, the precise contact zones with the Greek smooth newt and Schmidtler's smooth newt are not yet clear.[12] In central Italy, where the range of the smooth newt subspecies L. v. meridionalis overlaps with that of the Italian newt (L. italicus), it was found that the latter prefers a warmer and drier climate.[20]
Introduced range
The nominate subspecies, L. v. vulgaris, has been introduced to Australia, which has no native salamander species. The smooth newt was available in the Australian pet trade until 1997, when it was declared a "controlled pest animal" because of the risk of introduction. The first record in the wild was made near Melbourne in 2011, and larvae were later found, indicating successful reproduction. Negative impacts on the native fauna are feared, including predation on and competition with native frogs and freshwater invertebrates, toxicity, and disease spread. The smooth newt could spread further in south-eastern Australia, where wide areas have a suitable climate.[21]
Within Europe, the subspecies L. v. meridionalis was introduced north of the Alps near Geneva, where it hybridises with the native L. v. vulgaris.[22]
Habitats

Mainly a lowland species, the smooth newt is only exceptionally found above 1,000 m (3,300 ft).[11]:78–80 It accepts a wide range of terrestrial and aquatic habitats. On land, it occurs in wooded areas (dense conifer woods are avoided) but also in more open areas such as damp meadows, field edges, parks and gardens. It readily adapts to urban environments. The newts hide under structures such as logs or stones or in small mammal burrows.[11]:120–134[19][5]:238
Freshwater breeding sites must be close to the land habitats. They are typically sun-exposed, free from fish, stagnant, water-filled permanently or for at least three months of the year, close to similar water bodies, and have shallow areas with abundant water plants. They can range from small puddles to larger ponds or shallow parts of lakes. Water quality is less important; pH values from 4 (more acidic) to 9.6 (more alkaline) are tolerated and in Germany, smooth newts have even been found in slightly brackish water.[11]:121–129 They often share breeding sites with other amphibians, including other newts; in northern France, ponds with five newt species – smooth, palmate, alpine, northern crested and marbled (T. marmoratus) newt – have been described.[11]:151–152
Lifecycle and behaviour
Smooth newts live on land during most of the year and are mainly nocturnal. They also usually hibernate on land, often in congregations of several newts in winter shelters such as under logs or in burrows (but they can be active during mild weather[23]). The efts turn into mature adults at two to three years, and the newts can reach an age of 6–14 years in the wild.[5]:238 The newts recognise familiar territory using smell and visual cues, but could not orient themselves in experiments when they were transported far away from the home range.[24]
Reproduction
| External video | |
|---|---|

_(8618458053).jpg.webp)
Migration to the breeding sites occurs as soon as February, but in the northern parts of the range and at higher altitudes, it may not start before summer. After entering the water, the breeding characters, especially the male's crest, take a few weeks to develop.[5]:238
Mating involves an intricate courtship display: The male attempts to attract a female by swimming in front of her and sniffing her cloaca. He then vibrates his tail against his body, sometimes violently lashing it, thereby fanning pheromones towards her. In the final phase, he moves away from her, the tail quivering. If she is still interested, she will follow him and touch his cloaca with her snout, whereupon he deposits a packet of sperm (a spermatophore). He then guides her over the spermatophore so she picks it up with her cloaca. Males often try to lead females away from displaying competitors.[5]:238–240
Eggs are fertilised internally, and progeny of one female usually has multiple fathers. Females tend to mate preferentially with unrelated males, probably to avoid inbreeding depression.[25]
Females lay 100–500 eggs, usually folding them into waterplants. The eggs are 1.3–1.7 mm in diameter (2.7–4 mm with jelly capsule) and light brown to greenish or grey in colour. Larvae typically hatch after 10–20 days, depending on temperature, and metamorphose into terrestrial efts after around three months.[5]:238–240
Paedomorphism, where adults stay aquatic and retain their gills and skin seams or only resorb them partially, occurs regularly but only in a small proportion of individuals. It does not appear to be determined genetically but favoured by cold water, a low density of individuals and abundant aquatic prey. Wild paedomorphic individuals often metamorphosed when they were transferred into an aquarium.[11]:192–193
Diet, predators and parasites

Smooth newts, including the larvae, are unselective carnivores, feeding mainly on diverse invertebrates such as earthworms, snails or insects, or smaller plankton. Cannibalism also occurs, mainly by preying on eggs of its own species. Various predators eat smooth newts, including waterbirds, snakes and frogs, but also larger newts such as the northern crested newt.[5]:238
Various pathogens and parasites have been found to infect smooth newts, including ranaviruses,[26] a picornavirus,[27] various protozoans,[11]:164 trematodes[28][11]:164 (of which Parastrigea robusta was found to cause the local decline of a population in Germany[29]) and at least 31 species of helminths.[30]
Threats and conservation
The smooth newt is common over much of its range.[1][5]:237 The IUCN, in 2008, assessed its threat status as Least Concern and found no general decline in populations.[1] This assessment included subspecies now recognised as separate species (see section Taxonomy above) and needs updating.[12] Despite the overall low concern, the smooth newt is listed in some national red lists, e.g. in Switzerland, the Czech Republic, and the Netherlands.[11]:196 Like all amphibians, it is also listed as protected species in the Berne Convention (Appendix III).[31] Disturbance, capture, killing and trade are prohibited in Ireland under the Wildlife Act 1976,[32] and trade in the UK under the Wildlife and Countryside Act 1981.[33]
Threats to smooth newts are similar to those affecting other amphibians. They include especially the loss of breeding ponds through destruction or introduction of fish, and the fragmentation of population through roads.[11]:196–197 Secondary habitats can help sustain the species, e.g. former gravel pits or quarries left open.[11]:204–205 The value of artificial water bodies as habitat can be improved when nearby hiding structures like stones or wood are added on land.[34] Garden ponds are readily colonised if they are sun-exposed, have abundant water plants, no fish, and nearby hiding structures.[11]:206–218 Artificial hibernation sites ("newt hotels") were readily used in a study in Norway, especially by juveniles.[35]
To mark and track individuals and monitor populations, researchers have often amputated phalanges of fingers and toes but these re-grow quickly; a safer and less harmful alternative is recording the individual belly patterns through photography.[11]:223–224 Researchers have also developed genetic methods based on microsatellite distribution to assess patterns of genetic diversity.[36]
Captivity
Smooth newts can be kept in captivity, but must come from a legal source under the applicable legislation given their protected status (see above). They need a land and water phase, with hibernation for two to three months at 5–10 °C.[11]:210–215 The juveniles remain terrestrial and will only return to water at maturity. Individuals have reached ages of 4–8, exceptionally up to 20 years, in captivity.[5]:240
References
- Arntzen, J.W.; Kuzmin, S.; Beebee, T.; et al. (2009). "Lissotriton vulgaris". IUCN Red List of Threatened Species. 2009: e.T59481A11932252. doi:10.2305/IUCN.UK.2009.RLTS.T59481A11932252.en. Retrieved 1 November 2020.
- Pabijan, M.; Zieliński, P.; Dudek, K.; Stuglik, M.; Babik, W. (2017). "Isolation and gene flow in a speciation continuum in newts". Molecular Phylogenetics and Evolution. 116: 1–12. doi:10.1016/j.ympev.2017.08.003. ISSN 1055-7903. PMID 28797693.
- Frost, D.R. (2020). "Lissotriton vulgaris (Linnaeus, 1758)". Amphibian Species of the World: an Online Reference. Version 6.1. New York, USA: American Museum of Natural History. doi:10.5531/db.vz.0001. Retrieved 18 April 2020.
- Linnaeus, C. (1767). Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis (in Latin). 1 (part 1) (10 ed.). Stockholm, Sweden: L. Salvii. doi:10.5962/bhl.title.37256.
- Sparreboom, M. (2014). Salamanders of the Old World: The Salamanders of Europe, Asia and Northern Africa. Zeist, The Netherlands: KNNV Publishing. doi:10.1163/9789004285620. ISBN 9789004285620.
- Titus, T.A.; Larson, A. (1995). "A molecular phylogenetic perspective on the evolutionary radiation of the salamander family Salamandridae". Systematic Biology. 44 (2): 125–151. doi:10.1093/sysbio/44.2.125. ISSN 1063-5157.
- Weisrock, D.W.; Papenfuss, T.J.; Macey, J.R.; et al. (2006). "A molecular assessment of phylogenetic relationships and lineage accumulation rates within the family Salamandridae (Amphibia, Caudata)". Molecular Phylogenetics and Evolution. 41 (2): 368–383. doi:10.1016/j.ympev.2006.05.008. ISSN 1055-7903. PMID 16815049.
- Steinfartz, S.; Vicario, S.; Arntzen, J.W.; Caccone, A. (2007). "A Bayesian approach on molecules and behavior: reconsidering phylogenetic and evolutionary patterns of the Salamandridae with emphasis on Triturus newts". Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 308B (2): 139–162. doi:10.1002/jez.b.21119. ISSN 1552-5007. PMID 16969762.
- García-París, M.; Montori, A.; Herrero, P. (2004). Amphibia: Lissamphibia. Fauna Iberica. 24. Madrid: Museo Nacional de Ciencias Naturales, Consejo Superior de Investigaciones Científicas. ISBN 8400082923.
- Bell, T. (1839). A History of British Reptiles. London: John van Voorst. doi:10.5962/bhl.title.5498.
- Große, W-R. (2011). Der Teichmolch [The smooth newt]. Die neue Brehm-Bücherei (in German). 117. Magdeburg, Germany: VerlagsKG Wolf. ISBN 978-3-89432-476-6. ISSN 0138-1423.
- Wielstra, B.; Canestrelli, D.; Cvijanović, M.; et al. (2018). "The distributions of the six species constituting the smooth newt species complex (Lissotriton vulgaris sensu lato and L. montandoni) – an addition to the New Atlas of Amphibians and Reptiles of Europe" (PDF). Amphibia-Reptilia. 39 (2): 252–259. doi:10.1163/15685381-17000128.
- Zieliński, P.; Nadachowska-Brzyska, K.; Dudek, K.; Babik, W. (2016). "Divergence history of the Carpathian and smooth newts modelled in space and time". Molecular Ecology. 25 (16): 3912–3928. doi:10.1111/mec.13724. ISSN 0962-1083. PMID 27288862. S2CID 206183624.
- Pabijan, M.; Zieliński, P.; Dudek, K.; et al. (2015). "The dissection of a Pleistocene refugium: phylogeography of the smooth newt, Lissotriton vulgaris, in the Balkans". Journal of Biogeography. 42 (4): 671–683. doi:10.1111/jbi.12449. ISSN 0305-0270.
- Babik, W.; Branicki, W.; Crnobrnja-Isailovic, J.; et al. (2005). "Phylogeography of two European newt species – discordance between mtDNA and morphology". Molecular Ecology. 14 (8): 2475–2491. doi:10.1111/j.1365-294X.2005.02605.x. ISSN 0962-1083. PMID 15969729. S2CID 7484766.
- Skorinov, Dmitriy V.; Litvinchuk, Spartak N. (2016). "Tracing glacial refugia of the smooth newt (Lissotriton vulgaris) based on species distribution modelling". Vestnik of Saint Petersburg University. Biology. 3: 136–143. doi:10.21638/11701/spbu03.2016.323. ISSN 1025-8604.
- Modesti, Andrea; Aguzzi, Stefano; Manenti, Raoul (2011). "A case of complete albinism in Lissotriton vulgaris meridionalis" (PDF). Herpetology Notes. 4: 395–396. ISSN 2071-5773.
- Beebee, T & Griffiths, R. (2000) The New Naturalist: Amphibians and reptiles – a natural history of the British herpetofauna; Harper Collins Publishers, London.
- Kuzmin, S. (1999). "AmphibiaWeb – Lissotriton vulgaris". Archived from the original on 19 April 2019. Retrieved 26 April 2020.
- Iannella, Mattia; Cerasoli, Francesco; Biondi, Maurizio (2017). "Unraveling climate influences on the distribution of the parapatric newts Lissotriton vulgaris meridionalis and L. italicus". Frontiers in Zoology. 14 (1): 55. doi:10.1186/s12983-017-0239-4. ISSN 1742-9994. PMC 5727953. PMID 29255477.
- Tingley, R.; Weeks, A.R.; Smart, A.S.; et al. (2014). "European newts establish in Australia, marking the arrival of a new amphibian order" (PDF). Biological Invasions. 17 (1): 31–37. doi:10.1007/s10530-014-0716-z. hdl:11343/216887. ISSN 1387-3547. S2CID 18950725.
- Dubey, Sylvain; Lavanchy, Guillaume; Thiébaud, Jacques; Dufresnes, Christophe (2019). "Herps without borders: a new newt case and a review of transalpine alien introductions in western Europe". Amphibia-Reptilia. 40 (1): 13–27. doi:10.1163/15685381-20181028. ISSN 0173-5373.
- Kaczmarek, J. M.; Piasecka, M.; Kaczmarski, M. (2018). "Winter activity of the smooth newt Lissotriton vulgaris in Central Europe". The Herpetological Bulletin. 144: 21–22. ISSN 1473-0928.
- Sinsch, U.; Kirst, C. (2015). "Homeward orientation of displaced newts (Triturus cristatus, Lissotriton vulgaris) is restricted to the range of routine movements". Ethology Ecology & Evolution. 28 (3): 312–328. doi:10.1080/03949370.2015.1059893. ISSN 0394-9370. S2CID 83929007.
- Jehle, R.; Sztatecsny, M.; Wolf, J.B.W; et al. (2007). "Genetic dissimilarity predicts paternity in the smooth newt (Lissotriton vulgaris)". Biology Letters. 3 (5): 526–528. doi:10.1098/rsbl.2007.0311. PMC 2391198. PMID 17638673.
- Saucedo, Bernardo; Garner, Trenton W. J.; Kruithof, Natasja; et al. (2019). "Common midwife toad ranaviruses replicate first in the oral cavity of smooth newts (Lissotriton vulgaris) and show distinct strain-associated pathogenicity". Scientific Reports. 9 (1): 4453. doi:10.1038/s41598-019-41214-0. ISSN 2045-2322. PMC 6418247. PMID 30872735.
- Pankovics, Péter; Boros, Ákos; Tóth, Zoltán; et al. (2016). "Genetic characterization of a second novel picornavirus from an amphibian host, smooth newt (Lissotriton vulgaris)". Archives of Virology. 162 (4): 1043–1050. doi:10.1007/s00705-016-3198-8. ISSN 0304-8608. PMID 28005212. S2CID 531673.
- Caffara, M.; Bruni, G.; Paoletti, C.; Gustinelli, A.; Fioravanti, M.L. (2013). "Metacercariae of Clinostomum complanatum (Trematoda: Digenea) in European newts Triturus carnifex and Lissotriton vulgaris (Caudata: Salamandridae)". Journal of Helminthology. 88 (3): 278–285. doi:10.1017/S0022149X13000151. ISSN 0022-149X. PMID 23506789.
- Sinsch, Ulrich; Kaschek, Jacqueline; Wiebe, Jessica. "Heavy metacercariae infestation (Parastrigea robusta) promotes the decline of a smooth newt population (Lissotriton vulgaris)". Salamandra. 45 (3): 210–221. ISSN 0036-3375.
- Sinsch, U.; Heneberg, P.; Těšínský, M.; Balczun, C.; Scheid, P. (2018). "Helminth endoparasites of the smooth newt Lissotriton vulgaris: linking morphological identification and molecular data". Journal of Helminthology. 93 (3): 332–341. doi:10.1017/S0022149X18000184. ISSN 0022-149X. PMID 29502544.
- "Convention on the Conservation of European Wildlife and Natural Habitats". Bern: Council of Europe. 1979. Retrieved 1 November 2020.
- Nelson, B.; Cummins, S.; Fay, L.; et al. (2019). "Checklists of protected and rare species in Ireland" (PDF). Irish Wildlife Manuals. National Parks and Wildlife Service, Department of Culture, Heritage and the Gaeltacht. 116. ISSN 1393-6670. Retrieved 1 November 2020.
- "Wildlife and Countryside Act 1981". Article 9, Act of 1981. United Kingdom.
- Mulkeen, C.J.; Gibson-Brabazon, S.; Carlin, C.; et al. (2017). "Habitat suitability assessment of constructed wetlands for the smooth newt (Lissotriton vulgaris [Linnaeus, 1758]): A comparison with natural wetlands". Ecological Engineering. 106: 532–540. doi:10.1016/j.ecoleng.2017.06.005. ISSN 0925-8574.
- Dervo, Børre; Museth, Jon; Skurdal, Jostein (2018). "Assessing the use of artificial hibernacula by the great crested newt (Triturus cristatus) and smooth newt (Lissotriton vulgaris) in cold climate in southeast Norway". Diversity. 10 (3): 56. doi:10.3390/d10030056. ISSN 1424-2818.
- Buono, Vincenzo; Galliani, Giorgia; Mancini, Emiliano; et al. (2018). "An improved microsatellite panel to assess genetic variability of the Italian smooth newt (Lissotriton vulgaris meridionalis)". Journal of Genetics. 97 (2): 569–573. doi:10.1007/s12041-018-0934-8. hdl:11573/1278542. ISSN 0022-1333. PMID 29932078. S2CID 46977273.

