Southern stingray
The southern stingray (Hypanus americanus) is a whiptail stingray found in tropical and subtropical waters of the Western Atlantic Ocean from New Jersey to southern Brazil.[2] It has a flat, diamond-shaped disc, with a mud brown, olive, and grey dorsal surface and white underbelly (ventral surface).[3] The barb on its tail is serrated and covered in a venomous mucus, used for self-defense.
| Southern stingray | |
|---|---|
 | |
 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Chondrichthyes |
| Order: | Myliobatiformes |
| Family: | Dasyatidae |
| Genus: | Dasyatis |
| Species: | D. americanus |
| Binomial name | |
| Dasyatis americanus (Hildebrand & Schroeder, 1928) | |
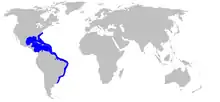 | |
| Range of the southern stingray | |
| Synonyms | |
| |
| Wikimedia Commons has media related to Hypanus americanus. |
Description

The southern stingray is adapted for life on the sea bed. The flattened, diamond-shaped body has sharp corners, making it more angular than the discs of other rays.[4] The top of the body varies between olive brown and green in adults, dark grey in juveniles, whilst the underside is predominantly white.[4][5] The wing-like pectoral fins are used to propel the stingray across the ocean bottom, whilst the slender tail possesses a long, serrated and venomous spine at the base, used for defence.[6] These spines are not fatal to humans, but are incredibly painful if stepped on. The eyes are situated on top of the head of the southern stingray, along with small openings called spiracles. The location of the spiracles enables the stingray to take in water whilst lying on the seabed, or when partially buried in sediment. Water enters the spiracles and leaves through the gill openings, bypassing the mouth which is on the underside.[4][6] Female stingrays can grow to a disc width of 150 cm, contrary to the smaller male stingrays that reach maximum size at 67 cm.[7][8]
Behavior
Southern stingrays are nocturnal predators, who spray water from their mouths or flap their fins vigorously to disturb the substrate and expose hidden prey. This bottom-dwelling species is often found singly or in pairs, and can reach population densities estimated up to 245 per km2 in certain shallow systems thought to be nursery grounds.[9] Hypanus americanus exhibit wave-like locomotion using their pectoral fins. This wave-like motion is important for Hypanus americanus because it allows them to escape predators, forage efficiently, and generally maneuver quickly. Typically, they travel large distances and their foraging area is very expansive. One study provided observations that Hypanus americanus swim along the tide, because of the greater food availability along tides. Hypanus americanus are able to do this because of their high maneuverability and efficient wave-like locomotion. Hypanus americanus either remain solitary or form groups. Groups of Hypanus americanus are usually observed when they mate, for predator protection or even when they are just resting.[10][11][12][13][14]
Foraging

In one study, it was evident that when scientists revealed the contents of the stomach of one Hypanus americanus, they found evidence of a great variety of ingested prey (which represented a variety of phyla and families), such as small fishes, worms and crustaceans. As mentioned earlier in this article, the Hypanus americanus swim with a wave-like motion, thus making it easier for them to maneuver and help explain why their foraging area is so vast. They can be identified as opportunistic feeders and continuous foragers, since they exhibit continuous feeding of multiple organisms throughout the day (this helps to explain the stomach contents revealed in the previously mentioned study).[15]
Predation

To avoid predators, Hypanus americanus bury and cover themselves in substrate. Their tails contain venom and are also utilized as protection from predators. Some predators of Hypanus americanus are humans and Hammerhead Sharks.[16][17]
Roles within their ecosystems
In shallow waters, there is a commensal foraging relationship between Hypanus americanus fish and Phalacrocorax auritus birds in coastal areas generally like the Gulf of Mexico. When foraging, the Hypanus americanus dig through the substrate in search of food; however, this also helps to expose certain other fish hidden in the substrate after which the Phalacrocorax auritus will follow behind the Hypanus americanus and eat.[18][19][20][21][22][23]
Reproduction
Hypanus americanus are ovoviviparous. Mother's bodies protect unborn offspring, while they are developing inside their mother's body. The embryos receive nutrients from the yolk sack early in development. Later in development, however, when the yolk sac is absorbed, the embryos obtain nutrients from the histotroph (the mother's uterine milk). After the Hypanus americanus offspring are born, and are outside of the mother's body, parental care ceases. In captivity, gestation lasted 135 to 226 days, after which a litter of two to ten young were born.[7]
There is little knowledge or published evidence about the mating systems of Hypanus americanus. Mating stingrays are rarely encountered in the wild. One study, however, does provide detailed observations of Hypanus americanus mating. This study involves observations of one female mating with two males. The study mentions that the female was chased by the two males, with one of the male's biting (or "catching") the female's fin. After copulation, the male releases the female from his bite on her. In addition, soon after giving birth, Hypanus americanus females have the ability to mate again.[24]
Sexual maturity and nursery type
_(San_Salvador_Island%252C_Bahamas)_7_(16152781622).jpg.webp)
Geographical location plays a large role in the age of sexual maturity. Observations from studies of breeding behavior (of Hypanus americanus during August at Bimini, Bahamas, and early September in Grand Cayman, Cayman Islands). One study shows that when females were placed in captivity, they were considered mature when they were impregnated (around 5 or 6 years old). In this case, males who were 3 or 4 years old were considered to be mature. There is also a difference in the rate at which the females bear young, depending on whether they are raised in captive natural environments or in natural environments. Females raised in captivity bear offspring twice a year, and females that are raised in the wild bear offspring once a year. In addition, there is a positive correlation between the size of the mother and the number of offspring.[24] There is a difference in nurseries for where the Hypanus americanus offspring are raised: there are primary and secondary nurseries which have a clear distinction. The primary nursery is defined as a habitat where a female Hypanus americanus gives birth to her young. On the other hand, the secondary nursery is a habitat where the juvenile Hypanus americanus are raised to mature adults. Little evidence about locations of and migrations between the primary and secondary nurseries is known. An example of a primary nursery is in Belize, where Hypanus americanus females pay seasonal visits for the purposes of mating and giving birth to offspring. During one study, juvenile Hypanus americanus were caught by scientists at 10 to 20 m depths on rock reef surfaces nearby during the months of May, November and December. This specific location of where these juvenile Hypanus americanus were collected was believed to be a secondary nursery.[7][25]
Communication
Studies of Hypanus americanus have shown that they communicate through pheromone signaling. Males communicate with females before copulating by touching and biting the females. Also, after the female gives birth, she releases pheromones that are most likely believed to be produced in her cloaca; one study reported that the birth of offspring attracted males. As previously mentioned in the article, since a female has the ability to mate soon after giving birth to her offspring, it is plausible that these are sex pheromones. The role of pheromones in communication also make sense since Hypanus americanus have strong senses of smell. They have many Ampullae of Lorenzini, usually heavily concentrated around the head. In addition, this gives them the ability to sense certain electric fields which are emitted from hidden prey. In addition, they have special mechanisms for senses vibrations in the water as well as for hearing.[24][26]
Human interaction

In many parts of the Caribbean such as Grand Cayman, Cayman Islands and Antigua, the southern stingray swims with divers and snorkelers, and are hand fed at locations such as Stingray City and the Sandbar.[13] On Turks & Caicos, they can be hand fed at a location called Gibbs Cay. Some have become tame enough to be cradled in visitors' arms and feed with pieces of cut up fish. This docile and food-reward driven behaviour has led to many locals comparing the hand-fed and belly-rubbed stingray to an over fed household canine. There are concerns that this feeding, and the high levels of interaction with humans, may be having some negative impacts on the behaviour and ecology of the stingrays.[27]
The southern stingray may make its way into the aquarium trade. Despite its relative hardiness, it is best avoided as it requires an immense 4,200 gallon capacity system and will devour any fish or invertebrate it is able to capture.[28] They are also housed within public aquariums and animal theme parks including Six Flags Discovery Kingdom in Vallejo, California and the Long Island Aquarium in Riverhead, New York where visitors are allowed to pet the rays in a touch pool.[29][30] In public aquariums, female southern stingrays have been seen biting one another on the edges of their fins. Reproduction has also been known to occur within large public aquariums.[28]
Gallery
 A Southern stingray in Bonaire.
A Southern stingray in Bonaire. Several Southern stingrays swimming around at Grand Cayman.
Several Southern stingrays swimming around at Grand Cayman..jpeg.webp) A Southern stingray in resting under a layer of sand in Costa Rica.
A Southern stingray in resting under a layer of sand in Costa Rica. A Southern stingray at the Georgia Aquarium in Atlanta, Georgia.
A Southern stingray at the Georgia Aquarium in Atlanta, Georgia._(San_Salvador_Island%252C_Bahamas)_12_(15533839933).jpg.webp) A Southern stingray resting near rock outcrops at San Salvador Island.
A Southern stingray resting near rock outcrops at San Salvador Island. A Southern stingray accompanied by a Rainbow runner (Elagatis bipinnulata) in Anegada.
A Southern stingray accompanied by a Rainbow runner (Elagatis bipinnulata) in Anegada._(36644323776).jpg.webp)
 The underside of a Southern stingray along with a few Yellowtail snapper (Ocyurus chrysurus).
The underside of a Southern stingray along with a few Yellowtail snapper (Ocyurus chrysurus).
References
This article incorporates text from the ARKive fact-file "Southern stingray" under the Creative Commons Attribution-ShareAlike 3.0 Unported License and the GFDL.
- Carlson, J., Charvet, P., Blanco-Parra, MP, Briones Bell-lloch, A., Cardenosa, D., Derrick, D., Espinoza, E., Morales-Saldaña, J.M., Naranjo-Elizondo, B., Pacoureau, N., Schneider, E.V.C., Simpson, N.J., Pollom, R. & Dulvy, N.K. (2020). "Hypanus americanus". The IUCN Red List of Threatened Species. IUCN. 2020: e.T181244884A104123787.CS1 maint: multiple names: authors list (link)
- Dasyatis americana Hildebrand & Schroeder, 1928. FishBase
- Bigelow, H. and Sschroder, W. (1953) Fishes of the Western North Atlantic. Sawfishes, guitarfishes, skates and rays. Mem. Sears Found. Mar. Res. 1, 1-558.
- Southern Stingray. Southern stingray Biological Profile, Ichthyology Department, Florida Museum of Natural History (August, 2007) - via ARKive
- Lieske, E. and Myers, R. (2002) Coral Reef Fishes: Indo-Pacific and Caribbean. HarperCollins Publishers, London – via ARKive
- Carpenter, K.E. (2001) The Living Marine Resources of the Western Central Atlantic, Volume 1. Food and Agriculture Organization of the United Nations, Rome – via ARKive
- Henningsen, A.D. (2000). "Notes on reproduction in the southern stingray, Dasyatis americana (Chondrichthyes: Dasyatidae), in a captive environment". Copeia. 0 (3): 826–828. doi:10.1643/0045-8511(2000)000[0826:NORITS]2.0.CO;2. JSTOR 1448353.
- McEachran, J. & de Carvalho, M. (2002) "Dasyatidae". In: K.E. Carpenter (ed.). The living marine resources of the Western Central Atlantic. Volume 1. Introduction, molluscs, crustaceans, hagfishes, sharks, batoid fishes and chimaeras. pp. 562–571.
- Tilley, Alexander; Strindberg, Samantha (2013). "Population density estimation of southern stingrays Dasyatis americanaon a Caribbean atoll using distance sampling". Aquatic Conservation: Marine and Freshwater Ecosystems. 23 (2): 202. doi:10.1002/aqc.2317.
- Semeniuk, Christina A. D.; Speers-Roesch, Ben; Rothley, Kristina D. (2007). "Using Fatty-Acid Profile Analysis as an Ecologic Indicator in the Management of Tourist Impacts on Marine Wildlife: A Case of Stingray-Feeding in the Caribbean". Environmental Management. 40 (4): 665–77. Bibcode:2007EnMan..40..665S. doi:10.1007/s00267-006-0321-8. PMID 17638047.
- Cartamil, D. P.; Vaudo, J. J.; Lowe, C. G.; Wetherbee, B. M.; Holland, K. N. (2003). "Diel movement patterns of the Hawaiian stingray, Dasyatis lata: implications for ecological interactions between sympatric elasmobranch species". Marine Biology. 142 (5): 841. doi:10.1007/s00227-003-1014-y.
- Rosenberger, L. (2001). "Pectoral fin locomotion in batoid fishes: Undulation versus oscillation" (PDF). The Journal of Experimental Biology. 204 (Pt 2): 379–94. PMID 11136623.
- Semeniuk, CAD; Rothley, KD (2008). "Costs of group-living for a normally solitary forager: effects of provisioning tourism on southern stingrays Dasyatis americana". Marine Ecology Progress Series. 357: 271–282. Bibcode:2008MEPS..357..271S. doi:10.3354/meps07299.
- Semeniuk, Christina A.D.; Haider, Wolfgang; Cooper, Andrew; Rothley, Kristina D. (2010). "A linked model of animal ecology and human behavior for the management of wildlife tourism". Ecological Modelling. 221 (22): 2699. doi:10.1016/j.ecolmodel.2010.07.018.
- Gilliam, D. & Sullivan, K. (1993). "Diet and feeding habits of the Southern stingray". Bulletin of Marine Science. 52 (3): 1007–1013.
- Passarelli, N., A. Piercy. (2006). "Southern Stingray". Florida Museum of Natural History. Accessed April 29, 2012
- Pikitch, E.; D. Chapman; E. Babcock; M. Shivji (2005). "Habitat use and demographic population structure of elasmobranchs at a Caribbean atoll (Glover's Reef, Belize)". Marine Ecology Progress Series. 302: 187–197. Bibcode:2005MEPS..302..187P. doi:10.3354/meps302187.
- Snelson, F.; S. Gruber; F. Murru; T. Schmid (1990). "Southern Stingray, Dasyatis americana: Host for a Symbiotic Cleaner Wrasse". Copeia. 1990 (4): 961–965. doi:10.2307/1446479. JSTOR 1446479.
- Sazima, C.; J. Krajewski; R. Bonaldo; I. Sazima (2007). "Nuclear-follower foraging associations of reef fishes and other animals at an oceanic archipelago". Environmental Biology of Fishes. 80 (4): 351–361. doi:10.1007/s10641-006-9123-3.
- Saunders, D. (1958). "The occurrence of Haemogregarina bigemina Laveran and Mesnil, and H. dasyatis n. sp. in marine fish from Bimini, Bahamas". Transactions of the American Microscopical Society. 77 (4): 404–412. doi:10.2307/3223863. JSTOR 3223863.
- Kohn, A.; S. Cohen; G. Salgado-Maldonado (2006). "Checklist of Monogenea parasites of freshwater and marine fishes, amphibians and reptiles from Mexico, Central America and Caribbean" (PDF). Zootaxa. 1289: 1–114.
- Kajiura, S.; L. Macesic; T. Meredith; K. Cocks; L. Dirk (2009). "Commensal Foraging Between Double-crested Cormorants and a Southern Stingray" (PDF). The Wilson Journal of Ornithology. 121 (3): 646–648. doi:10.1676/08-122.1.
- Brooks, D. & Mayes, M. (1980). "Cestodes in four species of euryhaline stingrays from Colombia". Proceedings of the Helminthological Society of Washington. 47: 22–29.
- Chapman, Demian D.; Corcoran, Mark J.; Harvey, Guy M.; Malan, Sonita; Shivji, Mahmood S. (2003). "Mating behavior of southern stingrays, Dasyatis americana (Dasyatidae)" (PDF). Environmental Biology of Fishes. 68 (3): 241. doi:10.1023/A:1027332113894.
- Henningsen, Alan D.; Leaf, Robert T. (2010). "Observations on the Captive Biology of the Southern Stingray". Transactions of the American Fisheries Society. 139 (3): 783. doi:10.1577/T09-124.1.
- Gardiner, J., R. Hueter, K. Maruska, J. Sisneros, B. Casper, D. Mann, L. Dernski. (2012). "Sensory Physiology and Behavior of Elasmobranchs", pp. 349-401 in J Carrier, J Musick, M Heithaus, eds. Biology of Sharks and Their Relatives, Second Edition. Boca Raton, FL: CRC Press.
- "Stingray Conservation and Ecology Research". cnso.nova.edu. Nova Southeastern University. Retrieved March 28, 2017.
- Michael, Scott (2001). Aquarium Sharks & Rays. Neptune City, NJ: T.F.H Publications, Inc.
External links
| Wikimedia Commons has media related to Southern stingray. |
- Dasyatis americana, Southern stingray at FishBase
- Dasyatis americana (Southern Stingray) at IUCN Red List
- Southern stingray media from ARKive

- Biological Profiles: Southern Stingray at Florida Museum of Natural History Ichthyology Department
- Stingray City, Cayman Islands
- Photos of Southern stingray on Sealife Collection
