Seagrass meadow
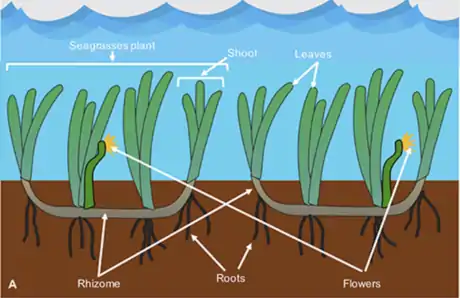
A seagrass meadow or seagrass bed is an underwater ecosystem formed by seagrasses. Seagrasses are marine (saltwater) plants found in shallow coastal waters and in the brackish waters of estuaries. Seagrasses are flowering plants with stems and long green, grass-like leaves. They produce seeds and pollen and have roots and rhizomes which anchor them in seafloor sand.
| Marine habitats |
|---|
 |
Seagrasses form dense underwater meadows which are among the most productive ecosystems in the world. They provide habitats and food for a diversity of marine life comparable to that of coral reefs. This includes invertebrates like shrimp and crabs, cod and flatfish, marine mammals and birds. They provide refuges for endangered species such as seahorses, turtles, and dulongs. They function as nursery habitats for shrimps, scallops and many commercial fish species. Seagrass meadows provide coastal storm protection by the way their leaves absorb energy from waves as they hit the coast. They keep coastal waters healthy by absorbing bacteria and nutrients, and slow the speed of climate change by sequestering carbon dioxide into the sediment of the ocean floor.
Seagrasses evolved from marine algae which colonized land and became land plants, and then returned to the ocean about 100 million years ago. However, today seagrass meadows are being damaged by human activities such as pollution from land runoff, fishing boats that drag dredges or trawls across the meadows uprooting the grass, and overfishing which unbalances the ecosystem. Seagrass meadows are currently being destroyed at a rate of about two football fields every hour.
Overview

Seagrasses are flowering plants that evolved from the land back to the sea, and now occupy the sea bottom in shallow waters along the coast all over the world. Seagrass meadows are hidden underwater grass fields that protect the coast and offer shelter to many sea creatures.[1]
Seagrasses are flowering plants (angiosperms) which grow in marine environments. There are about 60 species of fully marine seagrasses belonging to four families (Posidoniaceae, Zosteraceae, Hydrocharitaceae and Cymodoceaceae), all in the order Alismatales (in the class of monocotyledons).[2] Seagrasses evolved from terrestrial plants which migrated back into the ocean about 75 to 100 million years ago.[3][4]
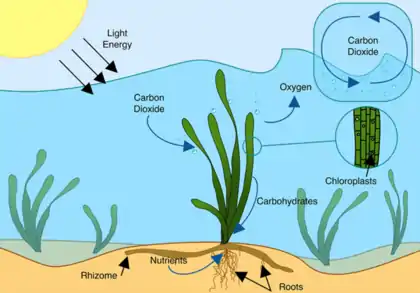
Like all autotrophic plants, seagrasses photosynthesize, in the submerged photic zone, and most occur in shallow and sheltered coastal waters anchored in sand or mud bottoms. Most species undergo submarine pollination and complete their life cycle underwater.
Seagrasses beds/meadows can be either monospecific (made up of a single species) or in mixed beds. In temperate areas, usually one or a few species dominate (like the eelgrass Zostera marina in the North Atlantic), whereas tropical beds usually are more diverse, with up to thirteen species recorded in the Philippines.
Seagrass meadows form in maximum depths of up to 50m, depending on water quality and light availability, and can include up to 12 different species in one meadow.[5] These seagrass meadows are highly productive habitats that provide many ecosystem services, including sediment stabilization, habitat and biodiversity, better water quality, and carbon and nutrient sequestration.[6]
Seagrass beds, sometimes called prairies of the sea, are diverse and productive ecosystems which harbour species from all phyla, for example juvenile and adult fish, epiphytic and free-living macroalgae and microalgae, mollusks, bristle worms, and nematodes. Few species were originally considered to feed directly on seagrass leaves (partly because of their low nutritional content), but scientific reviews and improved working methods have shown that seagrass herbivory is an important link in the food chain, feeding hundreds of species, including green turtles, dugongs, manatees, fish, geese, swans, sea urchins and crabs. Some fish species that visit/feed on seagrasses raise their young in adjacent mangroves or coral reefs.
Global distribution
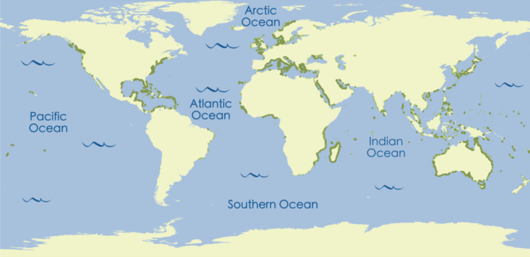
Seagrasses are found all over the world, in both hot and cold locations. Seagrasses live in shallow seas on the continental shelf of all continents except Antarctica. The continental shelf is the underwater area of land surrounding each continent, creating an area of relatively shallow water known as a shelf sea. It is believed that seagrasses cover 125,000 km2 around the world, but other estimates suggest that this number might be a lot bigger—seagrasses may cover up to 600,000 km2 of the shallow ocean.[7]
Seagrass meadows are found in areas with soft sediment that are either intertidal (uncovered daily by seawater, as the tide goes in and out) or subtidal (always under the water). Seagrasses prefer sheltered places, such as shallow bays, lagoons, and estuaries (sheltered areas where rivers flow in to the sea), where waves are limited and light and nutrient levels are high. Seagrasses can be found up to around 60 metres deep, but this depends on the availability of light because, like plants on the land, seagrass meadows need sunlight for photosynthesis to occur. Tides, wave action, water clarity, and low salinity (low amounts of salt in the water) control where seagrasses can live at their shallow edge nearest the shore,[9] all of these things must be just right for seagrass to survive and grow.[7]
The current documented seagrass area is 177,000 km2, but is thought to underestimate the total area since many areas with large seagrass meadows have not been thoroughly documented.[5] Most common estimates are 300,000 to 600,000 km2, with up to 4,320,000 km2 suitable seagrass habitat worldwide.[10]
Ecosystem services
_(South_Pigeon_Creek_estuary%252C_San_Salvador_Island%252C_Bahamas)_2_(16043820341).jpg.webp)
Seagrass meadows are one of the more important ecosystems. Seagrasses also cleanse the water of excess nutrients and toxic pollutants.
Although often overlooked, seagrasses provide coastal zones with a number of ecosystem goods and services. Seagrasses are considered ecosystem engineers.[12][4][3] This means that the plants alter the ecosystem around them. This adjusting occurs in both physical and chemical forms. Many seagrass species produce an extensive underground network of roots and rhizome which stabilizes sediment and reduces coastal erosion.[13] This system also assists in oxygenating the sediment, providing a hospitable environment for sediment-dwelling organisms.[12] Seagrasses also enhance water quality by stabilizing heavy metals, pollutants, and excess nutrients.[14][4][3] The long blades of seagrasses slow the movement of water which reduces wave energy and offers further protection against coastal erosion and storm surge. Furthermore, because seagrasses are underwater plants, they produce significant amounts of oxygen which oxygenate the water column. These meadows account for more than 10% of the ocean's total carbon storage. Per hectare, it holds twice as much carbon dioxide as rain forests and can sequester about 27.4 million tons of CO2 annually.[15] The storage of carbon is an essential ecosystem service as we move into a period of elevated atmospheric carbon levels.
Habitats for other species
 Stingray in seagrass
Stingray in seagrass Peppered moray in seagrass
Peppered moray in seagrass.jpg.webp) Blackspot emperor in seagrass
Blackspot emperor in seagrass



Nursery habitats
They provide nursery habitats for many commercially important fish species, and it's estimated that about half of the global fisheries get their start because they are supported by seagrass habitats. If these seagrass habitats are lost, then the fisheries are lost as well.
Coastal protection
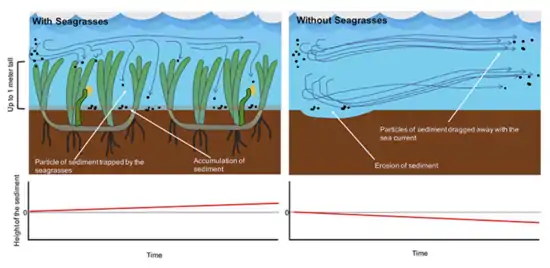
"Seagrasses are not only affected by water in motion, they also affect the currents, waves and turbulence environment is referred to as ecosystem engineering."(Jones et al., 1994, 1997; Thomas et al., 2000).[16]
Seagrasses prevent erosion of the seafloor to the point that their presence can raise the seafloor. Seagrasses contribute to coast protection by trapping rock debris transported by the sea. Seagrasses reduce erosion of the coast and protect houses and cities from both the force of the sea and from sea-level rise caused by global warming. Seagrasses do this by softening the force of the waves with their leaves, and helping sediment transported in the seawater to accumulate on the seafloor. Seagrass leaves act as baffles in turbulent water that slow down water movement and encourage particulate matter to settle out. Seagrass meadows are one of the most effective barriers against erosion, because they trap sediment amongst their leaves.[1]
Archaeologists have learned from seagrasses how to protect underwater archaeological sites, like a site in Denmark where dozens of ancient Roman and Viking shipwrecks have been discovered. The archaeologists use seagrass-like covers as sediment traps, to build up sediment so that it buries the ships. Burial creates low-oxygen conditions and keeps the wood from rotting.[17][1]
Support for fisheries
According to a 2019 paper by Unsworth et al,[18] the significant role seagrass meadows play in supporting fisheries productivity and food security across the globe is not adequately reflected in the decisions made by authorities with statutory responsibility for their management. They argue that: (1) Seagrass meadows provide valuable nursery habitat to over 1/5th of the world's largest 25 fisheries, including walleye pollock, the most landed species on the planet. (2) In complex small‐scale fisheries from around the world (poorly represented in fisheries statistics), there is evidence that many of those in proximity to seagrass are supported to a large degree by these habitats. (3) Intertidal fishing activity in seagrass is a global phenomenon, often directly supporting human livelihoods. According to the study, seagrasses should be recognized and managed to maintain and maximize their role in global fisheries production.[18]
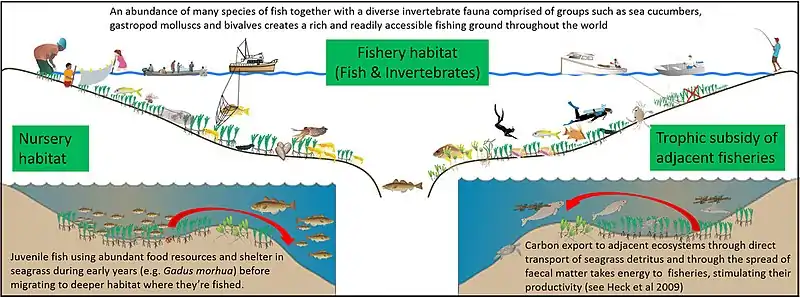

In the oceans, gleaning can be defined as fishing with basic gear, including bare hands, in shallow water not deeper than that one can stand.[20] Invertebrate gleaning (walking) fisheries are common within intertidal seagrass meadows globally, contributing to the food supply of hundreds of millions of people, but understanding of these fisheries and their ecological drivers are extremely limited. A 2019 study by Nessa et al. analysed these fisheries using a combined social and ecological approach. Catches were dominated by bivalves, sea urchins and gastropods. The catch per unit effort (CPUE) in all sites varied from 0.05 to 3 kg per gleaner per hour, with the majority of fishers being women and children. Landings were of major significance for local food supply and livelihoods at all sites. Local ecological knowledge suggests seagrass meadows are declining in line with other regional trends. Increasing seagrass density significantly and positively correlated with CPUE of the invertebrate gleaning (r = 0.830) highlighting the importance of conserving these threatened habitats.[19]
Blue carbon
.jpg.webp)
Blue carbon refers to carbon dioxide removed from the atmosphere by the world's coastal marine ecosystems, mostly mangroves, salt marshes, seagrasses and potentially macroalgae, through plant growth and the accumulation and burial of organic matter in the soil.[21][22]
Although seagrass makes up only 0.1% of the area of the ocean floor, it accounts for approximately 10-18% of the total oceanic carbon burial.[23] Currently global seagrass meadows are estimated to store as much as 19.9 Pg (gigaton, or billion tons) of organic carbon.[23] Carbon primarily accumulates in marine sediments, which are anoxic and thus continually preserve organic carbon from decadal-millennial time scales. High accumulation rates, low oxygen, low sediment conductivity and slower microbial decomposition rates all encourage carbon burial and carbon accumulation in these coastal sediments.[5] Compared to terrestrial habitats that lose carbon stocks as CO2 during decomposition or by disturbances like fires or deforestation, marine carbon sinks can retain C for much longer time periods. Carbon sequestration rates in seagrass meadows vary depending on the species, characteristics of the sediment, and depth of the habitats, but on average the carbon burial rate is approximately 138 g C m−2 yr−1.[14]
Biogeochemistry
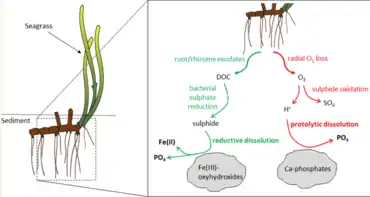
The primary nutrients determining seagrass growth are carbon (C), nitrogen (N), phosphorus (P), and light for photosynthesis. Nitrogen and phosphorus can be acquired from sediment pore water or from the water column, and sea grasses can uptake N in both ammonium (NH4+) and nitrate (NO3−) form.[25]
A number of studies from around the world have found that there is a wide range in the concentrations of C, N, and P in seagrasses depending on their species and environmental factors. For instance, plants collected from high-nutrient environments had lower C:N and C:P ratios than plants collected from low-nutrient environments. Seagrass stoichiometry does not follow the Redfield ratio commonly used as an indicator of nutrient availability for phytoplankton growth. In fact, a number of studies from around the world have found that the proportion of C:N:P in seagrasses can vary significantly depending on their species, nutrient availability, or other environmental factors. Depending on environmental conditions, seagrasses can be either P-limited or N-limited.[26]
An early study of seagrass stoichiometry suggested that the Redfield balanced ratio between N and P for seagrasses is approximately 30:1.[27] However, N and P concentrations are strictly not correlated, suggesting that seagrasses can adapt their nutrient uptake based on what is available in the environment. For example, seagrasses from meadows fertilized with bird excrement have shown a higher proportion of phosphate than unfertilized meadows. Alternately, seagrasses in environments with higher loading rates and organic matter diagenesis supply more P, leading to N-limitation. P availability in Thalassia testudinum is the limiting nutrient. The nutrient distribution in Thalassia testudinum ranges from 29.4-43.3% C, 0.88-3.96% N, and 0.048-0.243% P. This equates to a mean ratio of 24.6 C:N, 937.4 C:P, and 40.2 N:P. This information can also be used to characterize the nutrient availability of a bay or other water body (which is difficult to measure directly) by sampling the seagrasses living there.[28]
Light availability is another factor that can affect the nutrient stoichiometry of seagrasses. Nutrient limitation can only occur when photosynthetic energy causes grasses to grow faster than the influx of new nutrients. For example, low light environments tend to have a lower C:N ratio.[28] Alternately, high-N environments can have an indirect negative effect to seagrass growth by promoting growth of algae that reduce the total amount of available light.[29]
Nutrient variability in seagrasses can have potential implications for wastewater management in coastal environments. High amounts of anthropogenic nitrogen discharge could cause eutrophication in previously N-limited environments, leading to hypoxic conditions in the seagrass meadow and affecting the carrying capacity of that ecosystem.[28]
A study of annual deposition of C, N, and P from Posidonia oceanica seagrass meadows in northeast Spain found that the meadow sequestered 198 g C m−2 yr−1, 13.4 g N m−2 yr−1, and 2.01 g P m−2 yr−1 into the sediment. Subsequent remineralization of carbon from the sediments due to respiration returned approximately 8% of the sequestered carbon, or 15.6 g C m−2 yr −1.[30]
Human impacts
Human activities, such as fishing methods that rely on heavy nets that are dragged across the sea floor, put this important ecosystem at serious risk.[1] Seagrass habitats are threatened by coastal eutrophication and increased seawater temperatures,[5] as well as increased sedimentation and coastal development.[14] Seagrass loss has accelerated over the past few decades, from 0.9% per year prior to 1940 to 7% per year in 1990.[31]
 Seagrass lagoon, Chek Jawa, Singapore.
Seagrass lagoon, Chek Jawa, Singapore.
References
- Fusi M and Daffonchio D (2019) "How Seagrasses Secure Our Coastlines". Frontiers for Young Minds. 7: 114. doi:10.3389/frym.2019.00114.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Tomlinson and Vargo (1966). "On the morphology and anatomy of turtle grass, Thalassia testudinum (Hydrocharitaceae). I. Vegetative Morphology". Bulletin of Marine Science. 16: 748–761.
- Orth; et al. (2006). "A global crisis for seagrass ecosystems". BioScience. 56 (12): 987–996. doi:10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2. hdl:10261/88476.
- Papenbrock, J (2012). "Highlights in seagrass' phylogeny, physiology, and metabolism: what makes them so species?". International Scholarly Research Network: 1–15.
- Duarte, CM (2011). "Assessing the capacity of seagrass meadows for carbon burial: current limitations and future strategies". Ocean Coastal Management.
- Greiner, Jill (2013). "Seagrass restoration enhances "blue carbon" sequestration in coastal waters". PLOS ONE. 8 (8): e72469. Bibcode:2013PLoSO...872469G. doi:10.1371/journal.pone.0072469. PMC 3743776. PMID 23967303.
- Cullen-Unsworth, L.C., Jones, B.L., Lilley, R. and Unsworth, R.K. (2018) "Secret gardens under the sea: What are seagrass meadows and why are they important?" Frontiers for Young Minds, 6(2): 1–10. doi:10.3389/frym.2018.00002.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Reynolds PL (2018) "Seagrass and Seagrass Beds" Smithsonian Ocean Portal.
- Hemminga, M. A., and Duarte, C. M. (2000) Seagrass Ecology, first edition, Cambridge University Press. ISBN 9780521661843.
- Gattuso, J. (2006). "Light availability in the coastal ocean: impact on the distribution of benthic photosynthetic organisms and their contribution to primary production". Biogeosciences. 3 (4): 489–513. Bibcode:2006BGeo....3..489G. doi:10.5194/bg-3-489-2006.
- Dineen, J. (2001-07-25). "Thalassia testudinum (Turtle grass)". Smithsonian Marine Station at Fort Pierce. Retrieved 2012-11-07.
- Jones, Clive G.; Lawton, John H.; Shachak, Moshe (1994). "Organisms as ecosystem engineers". Oikos. 69 (3): 373–386. doi:10.2307/3545850. JSTOR 3545850.
- Grey, William; Moffler, Mark (1987). "Flowering of the seagrass Thalassia testudinum (Hydrocharitacea) in the Tampa Bay, Florida area". Aquatic Botany. 5: 251–259. doi:10.1016/0304-3770(78)90068-2.
- Darnell, Kelly; Dunton, Kenneth (2016). "Reproductive phenology of the subtropical seagrasses Thalassia testudinum (Turtle grass) and Halodule wrightii (Shoal grass) in the northwest Gulf of Mexico". Botanica Marina. 59 (6): 473–483. doi:10.1515/bot-2016-0080.
- Macreadie, P. I.; Baird, M. E.; Trevathan-Tackett, S. M.; Larkum, A. W. D.; Ralph, P. J. (2013). "Quantifying and modelling the carbon sequestration capacity of seagrass meadows". Marine Pollution Bulletin. 83 (2): 430–439. doi:10.1016/j.marpolbul.2013.07.038. PMID 23948090.
- Koch, E.W., Ackerman, J.D., Verduin, J. and van Keulen, M. (2007) "Fluid dynamics in seagrass ecology—from molecules to ecosystems". In" Seagrasses: biology, ecology and conservation, pages 193–225, Springer, Dordrecht. doi:10.1007/978-1-4020-2983-7_8.
- Gregory, D., Jensen, P. and Strætkvern, K. (2012) "Conservation and in situ preservation of wooden shipwrecks from marine environments". Journal of Cultural Heritage, 13(3): S139–S148. doi:10.1016/j.culher.2012.03.005.
- Unsworth, R.K., Nordlund, L.M. and Cullen‐Unsworth, L.C. (2019) "Seagrass meadows support global fisheries production". Conservation Letters, 12(1): e12566. doi:10.1111/conl.12566.

 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Nessa, N., Ambo-Rappe, R., Cullen-Unsworth, L.C. and Unsworth, R.K.F. (2019) "Social-ecological drivers and dynamics of seagrass gleaning fisheries". Ambio, pages 1–11. doi:10.1007/s13280-019-01267-x.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Nordlund, L.M., Unsworth, R.K., Gullström, M. and Cullen‐Unsworth, L.C. (2018) "Global significance of seagrass fishery activity. Fish and Fisheries", 19(3): 399–412. doi:10.1111/faf.12259. Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- Nellemann, Christian et al. (2009): Blue Carbon. The Role of Healthy Oceans in Binding Carbon. A Rapid Response Assessment. Arendal, Norway: UNEP/GRID-Arendal
- National Academies Of Sciences, Engineering (2019). Negative Emissions Technologies and Reliable Sequestration: A Research Agenda. Washington, D.C.: National Academies of Sciences, Engineering, and Medicine. p. 45. doi:10.17226/25259. ISBN 978-0-309-48452-7. PMID 31120708.
- Fourqurean, James W. (2012). "Seagrass ecosystems as a globally significant carbon stock". Nature Geoscience. 5 (7): 505–509. Bibcode:2012NatGe...5..505F. doi:10.1038/ngeo1477.
- Brodersen, K.E., Koren, K., Moßhammer, M., Ralph, P.J., Kühl, M. and Santner, J. (2017) "Seagrass-mediated phosphorus and iron solubilization in tropical sediments". Environmental science & technology, 51(24): 14155–14163. doi:10.1021/acs.est.7b03878.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Chung, I. K.; Beardall, J.; Mehta, S.; Sahoo, D.; Stojkovic, S. (2011). "Using marine macroalgae for carbon sequestration: a critical appraisal". Journal of Applied Phycology. 23 (5): 877–886. doi:10.1007/s10811-010-9604-9.
- Fourqurean, James W.; Zieman, Joseph C.; Powell, George V. N. (1992). "Phosphorus Limitation of Primary Production in Florida Bay: Evidence from C:N:P Ratios of the Dominant Seagrass Thalassia Testudinum". Limnology and Oceanography. 37 (1): 162–71. Bibcode:1992LimOc..37..162F. doi:10.4319/lo.1992.37.1.0162.
- Mcleod, E.; Chmura, G. L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C. M.; Silliman, B. R. (2011). "A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2" (PDF). Frontiers in Ecology and the Environment. 9 (10): 552–560. doi:10.1890/110004.
- Fourqurean, James W.; Zieman, Joseph C. (2002). "Nutrient Content of the Seagrass Thalassia Testudinum Reveals Regional Patterns of Relative Availability of Nitrogen and Phosphorus in the Florida Keys USA". Biogeochemistry. 61 (3): 229–45. doi:10.1023/A:1020293503405.
- Chmura, Gail; Anisfield, Shimon (2003). "Global carbon sequestration in tidal, saline wetland soils". Global Biogeochemical Cycles. 17 (4): n/a. Bibcode:2003GBioC..17.1111C. doi:10.1029/2002GB001917.
- Chi, Z.; Xie, Y.; Elloy, F.; Zheng, Y.; Hu, Y.; Chen, S. (2013). "Bicarbonate-based integrated carbon capture and algae production system with alkalihalophilic cyanobacterium". Bioresource Technology. 133: 513–521. doi:10.1016/j.biortech.2013.01.150. PMID 23455223.
- Waycott, M (2009). "Accelerating loss of seagrasses across the globe threatens coastal ecosystems". Proceedings of the National Academy of Sciences of the USA. 106 (30): 12377–12381. Bibcode:2009PNAS..10612377W. doi:10.1073/pnas.0905620106. PMC 2707273. PMID 19587236.
| Wikimedia Commons has media related to Seagrass meadows. |

_(southeastern_Graham's_Harbour%252C_San_Salvador_Island%252C_Bahamas)_2_(15428049493).jpg.webp)
_(South_Pigeon_Creek_estuary%252C_San_Salvador_Island%252C_Bahamas)_3_(15859724719).jpg.webp)
