Stramenopile
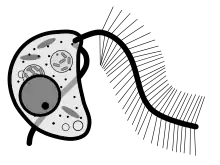
Stramenopile is a taxonomic concept that identifies a clade of organisms. A clade is conceived as any group of all of the descendants of a common ancestor (i.e. it is both holophyletic and monophyletic). A clade can be defined by reference to an evolutionarily innovative feature that the ancestor and its descendents share acknowledging that the character may be secondarily lost. The stramenopile clade is distinguished by the presence of stiff tripartite external hairs. In most species, the hairs are attached to flagella, in some they to other parts of the surface of cells, and in some they have been secondarily lost (in which relatedness to stramenopile ancestors is evident from other shared cytological features or from similarity in the makeup of genes).
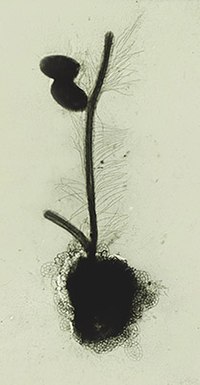
Members of the clade are referred to as 'stramenopiles'. Most stramenopiles are single celled eukaryotes (protists), but stramenopiles include some multicellular algae and fungi. The group includes a variety of algal protists, heterotrophic flagellates, opalines and closely related proteromonad flagellates (all endobionts in other organisms); the actinophryid heliozoa, and oomycetes. The tripartite hairs have been lost in some stramenopiles - for example in most diatoms (although they still make the proteins - see below).
History and the Heterokont problem
The term 'Stramenopile' was introduced in 1989.[1] It identified a group that overlapped with the ambiguously defined heterokonts (heterokont). The term 'Heterokontae" had been introduced 90 years earlier by Alexander Luther for algae that are now considered the Xanthophyceae.[2] The meaning of the term 'heterokont' was not stable. As an example, Copeland [3] used it to include the xanthophytes (using the name Vaucheriacea), a group that included what became known as the chrysophytes, the silicoflagellates, and the hyphochytrids. Copeland included unrelated collar flagellates (as the Choanoflagellata) in which he placed the bicosoecids, a type of stramenopile. He also included the haptophytes (Haptophyte). The consequence of multiple concepts for the taxon 'heterokont' was that the meaning could only be made clear by reference to its usage: Heterokontae sensu Luther 1899; Heterokonta sensu Copeland 1956, or heterokont in the sense of Heterokont Wkipedia on a particular date. The term 'Heterokont' lost its usefulness in critical discussions about the identity, nature, character and relatedness of the group.[4]
At the time of writing, the concept of the taxon Heterokont as used by some has evolved to align with that of Stramenopiles.

The presumed apomorphy of tripartite flagellar hairs is well characterized. The basal part of the hair is flexible and inserts into the cell membrane, the second part is dominated by a long stiff tube (the 'straw' ior 'stramen'), and the finally the tube is tipped by several delicate hairs [5]). The proteins that code for the hairs are also well characterized [6]> As far as we know, these proteins are exclusive to the stramenopile clade, and are present even in taxa (such as diatoms) that have no longer have physical hairs.
A taxonomic concept based on a synapomorphy is independent of the organisms that are included. Hence it is stable and robust as new findings may lead to the addition, elimination or regrouping of contained taxa. Such a concept would be invalidated if apomorphy in question were to be found to have evolved on more than one occasion. The term 'mastigoneme' refers to any kind of hair attached to protist flagella.)
Many stramenopiles are unicellular flagellates, and most others produce flagellated cells at some point in their lifecycles, for instance as gametes or zoospores. The term heterokont referred to the fact that the flagella of flagellated cells were dissimilar. The anterior flagellum has one or two rows of stiff hairs or mastigonemes. The posterior flagellum is without such embellishments, being smooth, usually shorter, or in a few cases not projecting from the cell. Opalines have many rows of flagella which do not have flagellar hairs. They are closely related to proteromonad flagellates some of which have tripartite hairs extending from the body surface.
The current composition is summarized as:
Stramenopiles without chloroplasts
- Oomycetes (water molds) inclusive of thraustrochytids
- Hyphochytridiomycetes
- Developayellids also referred to as Bigyromonadea
- Bicosoecea
- Labyrinthulomycetes (slime nets)
- Opalinesa
- Proteromonadsea
- Blastocystis
Stramenopiles with chloroplasts - Stramenochromes
- Axodines (Pedinellids and aplastidic actinophryid heliozoa)
- Bacillariophyceae (diatoms)
- Bolidophyceae
- Chrysomerophyceae
- Chrysophyceae (golden algae)
- Dictyochophyceae
- Eustigmatophyceae
- Pelagophyceae
- Phaeothamniophyceae
- Phaeophyceae (brown algae)
- Pinguiophyceae
- Raphidophyceae
- Synchromophyceae
- Synurophyceae
- Xanthophyceae (yellow-green algae)

The name of the taxon has also been written as Straminopiles, or more formally as Straminipila. The name "stramenopile" has been discussed by David (2002).[7]
In terms of relatedness among protists, Krylov and co-workers [8] proposed that the morphology of mitochondrial cristae remained relatively unchanged over time, and would define large sectors of protistan diversity. Subsequent molecular studies have confirmed this, with the stramenopiles being most closely related to Alveolates and Rhizaria - all with tubular mitochondrial cristae and collectively referred to as the SAR clade.[9]
Stramenochromes
The stramenopiles with plastids (stramenochromes)[10] have plastids with an off-green, orange, golden or brown color because of the occurrence of chlorophyll a, chlorophyll c, and fucoxanthin. That form of plastid is referred to as a chromoplast (as opposed to chloroplast which if used narrowly refers to the chlorophyll B containing plastids in green algae, some euglenids, and the land plants). The most significant autotrophic stramenopiles are the brown algae (wracks and many other seaweeds), and the ||diatom||s. The latter are among the most significant primary producers in marine and freshwater ecosystems. The flagella are inserted subapically or laterally, and are usually supported by four microtubule roots in a distinctive pattern.
Chromoplasts are surrounded by four membranes. The outermost is continuous with the chloroplast endoplasmic reticulum, or cER. The second membrane presents a barrier between the lumen of the cER and the primary endosymbiont or chloroplast. The symbiont was the source of the two innermost membranes within which the thylakoid membranes are found.
Most molecular analyses suggest that the most basal stramenopiles are colorless (heterotrophs) ([11] [12] This suggests that the stramenopiles arose as heterotrophic organisms, diversified, and only at a later stage in their evolutionary history did they acquire chloroplasts. Some lineages (such as the axodine lineage that included the chrophytic pedinellids, colourless ciliophryids, and colourless actinophryid heliozoa) have secondarily reverted to heterotrophy.
Significance
Some stramenopiles are significant as autotrophs and as heterotrophs in natural ecosystems. Blastocystis is a parasite of humans; opalines and proteromonads live in the intestines of cold-blooded vertebrates; oomycetes include some significant plant pathogens (inclusive of the agent of potato blight that caused the famine in Ireland that causes a million or so deaths and led to extensive emigration. Diatoms are major contributors to global carbon cycles, because they are the most important autotrophs in most marine habitats. The brown algae (or kelp) are major autotrophs of the interidal and subtidal marine habitats. Some of the bacterivorous stramenopiles, such as Cafeteria are common and widespread consumers of bacteria - and as such play a major role in recycling of carbon and nutrients with microbial food webs.
Gallery
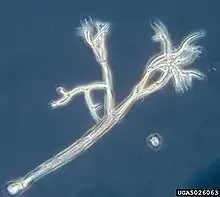
 Anthophysa (Stramenopile)
Anthophysa (Stramenopile).jpg.webp) Paraphysomonas (Stramenopile)
Paraphysomonas (Stramenopile)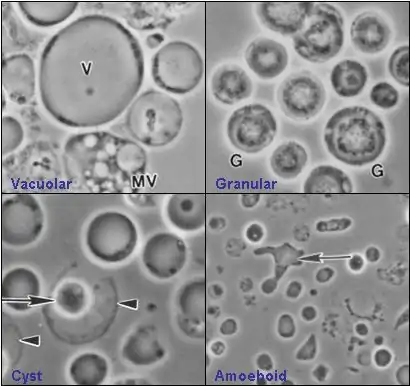 Blastocystis hominis (Blastocystea)
Blastocystis hominis (Blastocystea) Zelleriella (Opalinea)
Zelleriella (Opalinea)_Microscopy.tif.jpg.webp)
 Potatoes with Phytophthora (Oomycetes)
Potatoes with Phytophthora (Oomycetes)
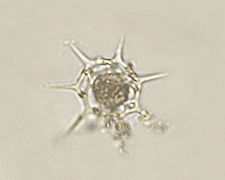
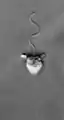 Actinomonas (Stramenopile)
Actinomonas (Stramenopile)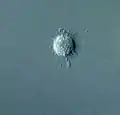 Ciliophrys (Stramenopile)
Ciliophrys (Stramenopile).jpg.webp) Actinophrys sol (Actinophryida)
Actinophrys sol (Actinophryida)
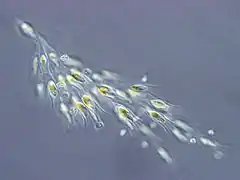 Dinobryon sp. (Chrysophyceae)
Dinobryon sp. (Chrysophyceae)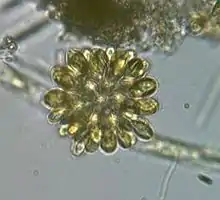 Synura sp. (Synurophyceae)
Synura sp. (Synurophyceae)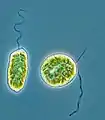 Haramonas (Raphidophyceae)
Haramonas (Raphidophyceae) Ophiocytium (Xanthophyta)
Ophiocytium (Xanthophyta) Fucus vesiculosus (Phaeophyceae)
Fucus vesiculosus (Phaeophyceae)
References
- Patterson, D. J. 1989. Stramenopiles: chromophytes from a protistological perspective. In: Green, J.C., Leadbeater, B.S.C. & Diver, W. L. 1989. The chromophyte algae: problems and perspectives. Clarendon Press, Oxford. 357-379
- Luther, Alexander F. (1899). Über Chlorosaccus eine neue Gattung der Süsswasseralgen nebst einiger Bemerkungen zur Systematik verwandter Algen. Stockholm: Norstedt. p. 1-22.
- Copeland, H. F. 1956. The Classification of Lower organisms. Pacific Books, Palo Alto, California
- Blackwell, W. H. (2009). "Chromista revisited: A dilemma of overlapping putative kingdoms, and the attempted application of the botanical code of nomenclature" (PDF). Phytologia. 91 (2).
- Bouck, G.B. 1971. The structure, origin, and comnposition of the tubular mastigonemes of the Ochromonas flagellum. J. Cell. Biol., 50: 362-384
- Blackman, L.M., Arikawa, M., Yamada, S., Suzaki, T., & Hardham, A. R. 2011. Identification of a Mastigoneme Protein from Phytophthora nicotianae. Protist 162: 100-114, https://doi.org/10.1016/j.protis.2010.01.005.
- David, J.C. 2002. A preliminary catalogue of the names of fungi above the rank of order. Constancea. 83: 1–30
- Krylov, M. V., A. A. Dobrovolskii, I. V. Issi, B. I. Michaelevich, S. A. Podlipaev, V. V. Reshetnyak, L. N. Seravin, et al. 1980. New concepts for the system of unicellular organisms. Trudy Zoologischkei Institut Akademiya Na�yuk, SSSR 94:122–132
- Derelle R, López-García P, Timpano H, Moreira D. A Phylogenomic Framework to Study the Diversity and Evolution of Stramenopiles (= Heterokonts). Mol Biol Evol. 2016 Nov;33(11):2890-2898. doi: 10.1093/molbev/msw168. Epub 2016 Aug 10. PMID: 27512113; PMCID: PMC5482393.
- Leipe, D. D., P. O. Wainright, J. H. Gunderson, D. Porter, D. J. Patterson, F. Valois, S. Himmerich and M. L. Sogin. 1994. The stramenopiles from a molecular perspective: 16S-like rRNA sequences from Labyrinthuloides minuta and Cafeteria roenbergensis. Phycologia 33: 369-377
- Leyland, B., Leu, S., Boussiba, S. 2017. Are Thraustochytrids algae? Fungal Biology,121:835-840
- Derelle R, López-García P, Timpano H, Moreira D. A Phylogenomic Framework to Study the Diversity and Evolution of Stramenopiles (= Heterokonts). Mol Biol Evol. 2016 Nov;33(11):2890-2898. doi: 10.1093/molbev/msw168. Epub 2016 Aug 10. PMID: 27512113; PMCID: PMC5482393.