Synthetic biological circuit
Synthetic biological circuits are an application of synthetic biology where biological parts inside a cell are designed to perform logical functions mimicking those observed in electronic circuits. The applications range from simply inducing production to adding a measurable element, like GFP, to an existing natural biological circuit, to implementing completely new systems of many parts.[1]
| Part of a series of articles on |
| Synthetic biology |
|---|
| Synthetic biological circuits |
| Genome editing |
| Artificial cells |
| Xenobiology |
| Other topics |
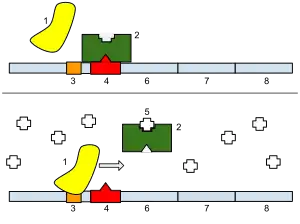
1: RNA polymerase, 2: Repressor, 3: Promoter, 4: Operator, 5: Lactose, 6: lacZ, 7: lacY, 8: lacA.
The goal of synthetic biology is to generate an array of tunable and characterized parts, or modules, with which any desirable synthetic biological circuit can be easily designed and implemented.[2] These circuits can serve as a method to modify cellular functions, create cellular responses to environmental conditions, or influence cellular development. By implementing rational, controllable logic elements in cellular systems, researchers can use living systems as engineered "biological machines" to perform a vast range of useful functions.[1]
History
The first natural gene circuit studied in detail was the lac operon. In studies of diauxic growth of E. coli on two-sugar media, Jacques Monod and Francois Jacob discovered that E.coli preferentially consumes the more easily processed glucose before switching to lactose metabolism. They discovered that the mechanism that controlled the metabolic "switching" function was a two-part control mechanism on the lac operon. When lactose is present in the cell the enzyme β-galactosidase is produced to convert lactose into glucose or galactose. When lactose is absent in the cell the lac repressor inhibits the production of the enzyme β-galactosidase to prevent any inefficient processes within the cell.
The lac operon is used in the biotechnology industry for production of recombinant proteins for therapeutic use. The gene or genes for producing an exogenous protein are placed on a plasmid under the control of the lac promoter. Initially the cells are grown in a medium that does not contain lactose or other sugars, so the new genes are not expressed. Once the cells reach a certain point in their growth, Isopropyl β-D-1-thiogalactopyranoside (IPTG) is added. IPTG, a molecule similar to lactose, but with a sulfur bond that is not hydrolyzable so that E. Coli does not digest it, is used to activate or "induce" the production of the new protein. Once the cells are induced, it is difficult to remove IPTG from the cells and therefore it is difficult to stop expression.
Two early examples of synthetic biological circuits were published in Nature in 2000. One, by Tim Gardner, Charles Cantor, and Jim Collins working at Boston University, demonstrated a "bistable" switch in E. coli. The switch is turned on by heating the culture of bacteria and turned off by addition of IPTG. They used GFP as a reporter for their system.[3] The second, by Michael Elowitz and Stanislas Leibler, showed that three repressor genes could be connected to form a negative feedback loop termed the Repressilator that produces self-sustaining oscillations of protein levels in E. coli.[4]
Currently, synthetic circuits are a burgeoning area of research in systems biology with more publications detailing synthetic biological circuits published every year.[5] There has been significant interest in encouraging education and outreach as well: the International Genetically Engineered Machines Competition[6] manages the creation and standardization of BioBrick parts as a means to allow undergraduate and high school students to design their own synthetic biological circuits.
Interest and goals
Both immediate and long term applications exist for the use of synthetic biological circuits, including different applications for metabolic engineering, and synthetic biology. Those demonstrated successfully include pharmaceutical production,[7] and fuel production.[8] However methods involving direct genetic introduction are not inherently effective without invoking the basic principles of synthetic cellular circuits. For example, each of these successful systems employs a method to introduce all-or-none induction or expression. This is a biological circuit where a simple repressor or promoter is introduced to facilitate creation of the product, or inhibition of a competing pathway. However, with the limited understanding of cellular networks and natural circuitry, implementation of more robust schemes with more precise control and feedback is hindered. Therein lies the immediate interest in synthetic cellular circuits.
Development in understanding cellular circuitry can lead to exciting new modifications, such as cells which can respond to environmental stimuli. For example, cells could be developed that signal toxic surroundings and react by activating pathways used to degrade the perceived toxin.[9] To develop such a cell, it is necessary to create a complex synthetic cellular circuit which can respond appropriately to a given stimulus.
Given synthetic cellular circuits represent a form of control for cellular activities, it can be reasoned that with complete understanding of cellular pathways, "plug and play"[1] cells with well defined genetic circuitry can be engineered. It is widely believed that if a proper toolbox of parts is generated,[10] synthetic cells can be developed implementing only the pathways necessary for cell survival reproduction. From this cell, to be thought of as a minimal genome cell, one can add pieces from the toolbox to create a well defined pathway with appropriate synthetic circuitry for an effective feedback system. Because of the basic ground up construction method, and the proposed database of mapped circuitry pieces, techniques mirroring those used to model computer or electronic circuits can be used to redesign cells and model cells for easy troubleshooting and predictive behavior and yields.
Example circuits
Oscillators
- Repressilator
- Mammalian tunable synthetic oscillator
- Bacterial tunable synthetic oscillator
- Coupled bacterial oscillator
- Globally coupled bacterial oscillator
Elowitz et al. and Fung et al. created oscillatory circuits that use multiple self-regulating mechanisms to create a time-dependent oscillation of gene product expression.[11][12]
Bistable switches
- Toggle-switch
Gardner et al. used mutual repression between two control units to create an implementation of a toggle switch capable of controlling cells in a bistable manner: transient stimuli resulting in persistent responses.[3]
Logical operators
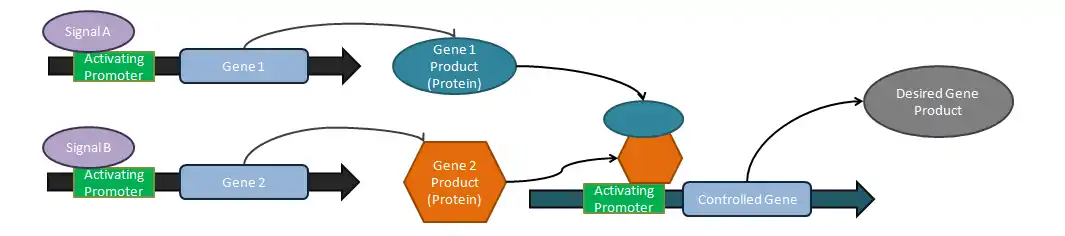
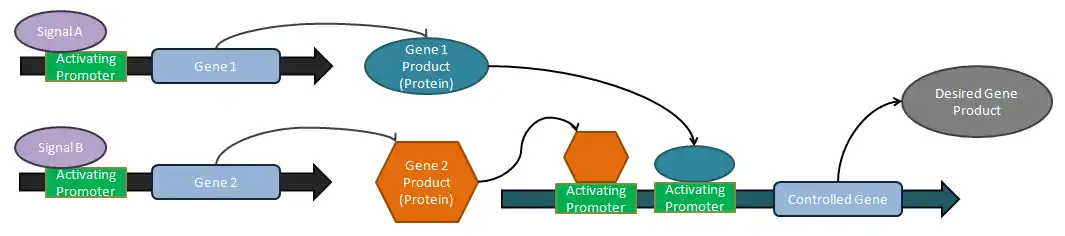
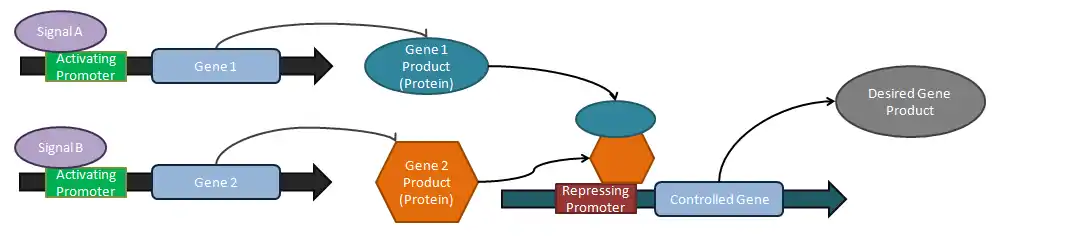
Analog tuners
Using negative feedback and identical promoters, linearizer gene circuits can impose uniform gene expression that depends linearly on extracellular chemical inducer concentration.[15]
Controllers of gene expression heterogeneity
Synthetic gene circuits can control gene expression heterogeneity can be controlled independently of the gene expression mean.[16]
Other engineered systems
Engineered systems are the result of implementation of combinations of different control mechanisms. A limited counting mechanism was implemented by a pulse-controlled gene cascade[17] and application of logic elements enables genetic "programming" of cells as in the research of Tabor et al., which synthesized a photosensitive bacterial edge detection program.[18]
Circuit design
Recent developments in artificial gene synthesis and the corresponding increase in competition within the industry have led to a significant drop in price and wait time of gene synthesis and helped improve methods used in circuit design.[19] At the moment, circuit design is improving at a slow pace because of insufficient organization of known multiple gene interactions and mathematical models. This issue is being addressed by applying computer-aided design (CAD) software to provide multimedia representations of circuits through images, text and programming language applied to biological circuits.[20] Some of the more well known CAD programs include GenoCAD, Clotho framework and j5.[21][22][23] GenoCAD uses grammars, which are either opensource or user generated "rules" which include the available genes and known gene interactions for cloning organisms. Clotho framework uses the Biobrick standard rules.[20]
References
- Kobayashi, H.; Kærn, M.; Araki, M.; Chung, K.; Gardner, T. S.; Cantor, C. R.; Collins, J. J. (2004). "Programmable cells: Interfacing natural and engineered gene networks". PNAS. 101 (22): 8414–8419. Bibcode:2004PNAS..101.8414K. doi:10.1073/pnas.0402940101. PMC 420408. PMID 15159530.
- "Synthetic Biology: FAQ". SyntheticBiology.org. Archived from the original on 12 December 2002. Retrieved 21 December 2011.
- Gardner, T.s., Cantor, C.R., Collins, J. Construction of a genetic toggle switch in Escherichia coli. Nature 403, 339-342 (20 January 2000).
- Stanislas Leibler; Elowitz, Michael B. (January 2000). "A synthetic oscillatory network of transcriptional regulators". Nature. 403 (6767): 335–338. Bibcode:2000Natur.403..335E. doi:10.1038/35002125. ISSN 1476-4687. PMID 10659856. S2CID 41632754.
- Purnick, Priscilla E. M.; Weis, Ron (2009). "The second wave of synthetic biology: from modules to systems". Nature Reviews Molecular Cell Biology. 10 (6): 410–422. doi:10.1038/nrm2698. PMID 19461664. S2CID 200495.
- International Genetically Engineered Machines (iGem) http://igem.org/Main_Page
- Ro, D.-K.; Paradise, E.M.; Ouellet, M.; Fisher, K.J.; Newman, K.L.; Ndungu, J.M.; Ho, K.A.; Eachus, R.A.; Ham, T.S.; Kirby, J.; Chang, M.C.Y.; Withers, S.T.; Shiba, Y.; Sarpong, R.; Keasling, J.D. (2006). "Production of the antimalarial drug precursor artemisinic acid in engineered yeast". Nature. 440 (7086): 940–943. Bibcode:2006Natur.440..940R. doi:10.1038/nature04640. PMID 16612385. S2CID 3199654.
- Fortman, J.L.; Chhabra, S.; Mukhopadhyay, A.; Chou, H.; Lee, T.S.; Steen, E.; Keasling, J.D. (2008). "Biofuel alternatives to ethanol: pumping the microbial well". Trends Biotechnol. 26 (7): 375–381. doi:10.1016/j.tibtech.2008.03.008. PMID 18471913.
- Keasling, J.D. (2008). "Synthetic biology for synthetic chemistry". ACS Chem Biol. 3 (1): 64–76. doi:10.1021/cb7002434. PMID 18205292.
- Lucks, Julius B; Qi, Lei; Whitaker, Weston R; Arkin, Adam P (2008). "Toward scalable parts families for predictable design of biological circuits". Current Opinion in Microbiology. 11 (6): 567–573. doi:10.1016/j.mib.2008.10.002. PMID 18983935.
- Elowitz, M.B.; Leibler, S. (2000). "A synthetic oscillatory network of transcriptional regulators". Nature. 403 (6767): 335–338. Bibcode:2000Natur.403..335E. doi:10.1038/35002125. PMID 10659856. S2CID 41632754.
- Fung, E.; Wong, W.W.; Suen, J.K.; Bulter, T.; Lee, S.; Liao, J.C. (2005). "A synthetic gene–metabolic oscillator". Nature. 435 (7038): 118–122. Bibcode:2005Natur.435..118F. doi:10.1038/nature03508. PMID 15875027. S2CID 414371.
- Silva-Rocha, R.; de Lorenzo, V. (2008). "Mining logic gates in prokaryotic transcriptional regulation networks". FEBS Letters. 582 (8): 1237–1244. doi:10.1016/j.febslet.2008.01.060. PMID 18275855. S2CID 45553956.
- Buchler, N.E.; Gerland, U.; Hwa, T. (2003). "On schemes of combinatorial transcription logic". PNAS. 100 (9): 5136–5141. Bibcode:2003PNAS..100.5136B. doi:10.1073/pnas.0930314100. PMC 404558. PMID 12702751.
- Nevozhay D, Adams RM, Murphy KF, Josic K, Balázsi G (March 31, 2009). "Negative autoregulation linearizes the dose-response and suppresses the heterogeneity of gene expression". Proc. Natl. Acad. Sci. U.S.A. 106 (13): 5123–8. Bibcode:2009PNAS..106.5123N. doi:10.1073/pnas.0809901106. PMC 2654390. PMID 19279212.
- Blake WJ, Balázsi G, Kohanski MA, Isaacs FJ, Murphy KF, Kuang Y, Cantor CR, Walt DR, Collins JJ (December 28, 2006). "Phenotypic Consequences of Promoter-Mediated Transcriptional Noise". Mol. Cell. 24 (6): 853–65. doi:10.1016/j.molcel.2006.11.003. PMID 17189188.
- Friedland, A.E.; Lu, T.K; Wang, X.; Shi, D.; Church, G.; Collins, J.J. (2009). "Synthetic Gene Networks That Count". Science. 324 (5931): 1199–1202. Bibcode:2009Sci...324.1199F. doi:10.1126/science.1172005. PMC 2690711. PMID 19478183.
- Tabor, J.J.; Salis, H.M.; Simpson, Z.B.; Chevalier, A.A.; Levskaya, A.; Marcotte, E.M.; Voigt, C.A.; Ellington, A.D. (2009). "A Synthetic Edge Detection Program". Cell. 137 (7): 1272–1281. doi:10.1016/j.cell.2009.04.048. PMC 2775486. PMID 19563759.
- Cheng, Allen A.; Lu, Timothy K. (2012-01-01). "Synthetic Biology: An Emerging Engineering Discipline". Annual Review of Biomedical Engineering. 14 (1): 155–178. doi:10.1146/annurev-bioeng-071811-150118. PMID 22577777. S2CID 7319630.
- Lux, Matthew W.; Bramlett, Brian W.; Ball, David A.; Peccoud, Jean (February 2012). "Genetic Design Automation: Engineering Fantasy or Scientific Renewal?". Trends in Biotechnology. 30 (4): 120–126. doi:10.1016/j.tibtech.2011.01.001. PMC 3073767. PMID 21310501.
- "GenoCAD: CAD Software for Synthetic Biology". www.genocad.com. Retrieved 2015-10-21.
- "Clotho". www.clothocad.org. Retrieved 2015-10-21.
- "J5". j5.jbei.org. Retrieved 2015-10-21.
