Protocell
A protocell (or protobiont) is a self-organized, endogenously ordered, spherical collection of lipids proposed as a stepping-stone toward the origin of life.[1][2] A central question in evolution is how simple protocells first arose and how they could differ in reproductive output, thus enabling the accumulation of novel biological emergences over time, i.e. biological evolution. Although a functional protocell has not yet been achieved in a laboratory setting, the goal to understand the process appears well within reach.[3][4][5][6]
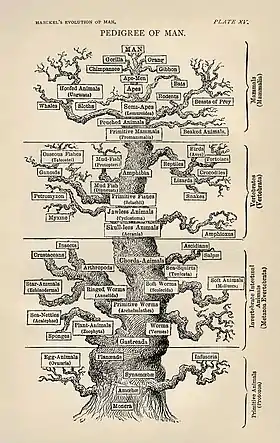
| Part of a series on |
| Evolutionary biology |
|---|
 |
|
Overview
Compartmentalization was important in the origins of life. Membranes form enclosed compartments that are separate from the external environment, thus providing the cell with functionally specialized aqueous spaces. As the lipid bilayer of membranes is impermeable to most hydrophilic molecules (dissolved by water), cells have membrane transport-systems that achieve the import of nutritive molecules as well as the export of waste.[7] It is very challenging to construct protocells from molecular assemblies. An important step in this challenge is the achievement of vesicle dynamics that are relevant to cellular functions, such as membrane trafficking and self-reproduction, using amphiphilic molecules. On the primitive Earth, numerous chemical reactions of organic compounds produced the ingredients of life. Of these substances, amphiphilic molecules might be the first player in the evolution from molecular assembly to cellular life.[8][9] A step from vesicle toward protocell might be to develop self-reproducing vesicles coupled with the metabolic system.[10]
Another approach to the notion of a protocell concerns the term "chemoton" (short for 'chemical automaton') which refers to an abstract model for the fundamental unit of life introduced by Hungarian theoretical biologist Tibor Gánti.[11] It is the oldest known computational abstract of a protocell. Gánti conceived the basic idea in 1952 and formulated the concept in 1971 in his book The Principles of Life (originally written in Hungarian, and translated to English only in 2003). He surmised the chemoton as the original ancestor of all organisms, or the last universal common ancestor.[12]
The basic assumption of the chemoton model is that life should fundamentally and essentially have three properties: metabolism, self-replication, and a bilipid membrane.[13] The metabolic and replication functions together form an autocatalytic subsystem necessary for the basic functions of life, and a membrane encloses this subsystem to separate it from the surrounding environment. Therefore, any system having such properties may be regarded as alive, and it will be subjected to natural selection and contain a self-sustaining cellular information. Some consider this model a significant contribution to origin of life as it provides a philosophy of evolutionary units.[14]
Selectivity for compartmentalization
Self-assembled vesicles are essential components of primitive cells.[1] The second law of thermodynamics requires that the universe move in a direction in which disorder (or entropy) increases, yet life is distinguished by its great degree of organization. Therefore, a boundary is needed to separate life processes from non-living matter.[15] The cell membrane is the only cellular structure that is found in all of the cells of all of the organisms on Earth.[16]
Researchers Irene A. Chen and Jack W. Szostak (Nobel Prize in Physiology or Medicine 2009) amongst others, demonstrated that simple physicochemical properties of elementary protocells can give rise to simpler conceptual analogues of essential cellular behaviors, including primitive forms of Darwinian competition and energy storage. Such cooperative interactions between the membrane and encapsulated contents could greatly simplify the transition from replicating molecules to true cells.[4] Competition for membrane molecules would favor stabilized membranes, suggesting a selective advantage for the evolution of cross-linked fatty acids and even the phospholipids of today.[4] This micro-encapsulation allowed for metabolism within the membrane, exchange of small molecules and prevention of passage of large substances across it.[17] The main advantages of encapsulation include increased solubility of the cargo and creating energy in the form of chemical gradient. Energy is thus often said to be stored by cells in the structures of molecules of substances such as carbohydrates (including sugars), lipids, and proteins, which release energy when chemically combined with oxygen during cellular respiration.[18][19]
Energy gradient
A March 2014 study by NASA's Jet Propulsion Laboratory demonstrated a unique way to study the origins of life: fuel cells.[20] Fuel cells are similar to biological cells in that electrons are also transferred to and from molecules. In both cases, this results in electricity and power. The study states that one important factor was that the Earth provides electrical energy at the seafloor. "This energy could have kick-started life and could have sustained life after it arose. Now, we have a way of testing different materials and environments that could have helped life arise not just on Earth, but possibly on Mars, Europa and other places in the Solar System."[20]
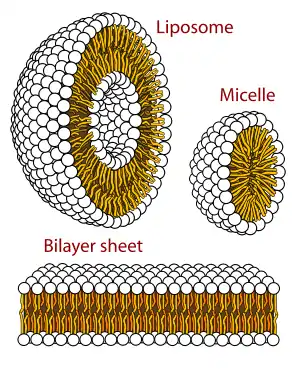
Vesicles, micelles and membraneless droplets
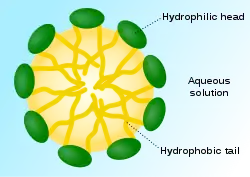
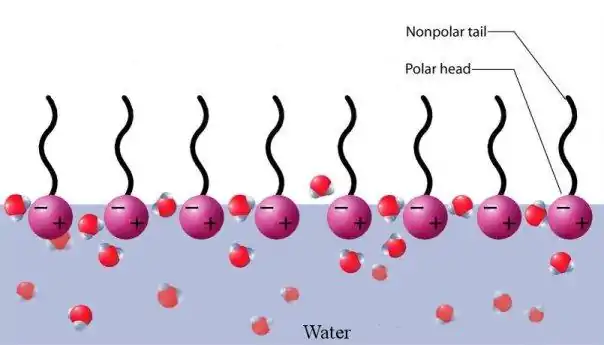
When phospholipids are placed in water, the molecules spontaneously arrange such that the tails are shielded from the water, resulting in the formation of membrane structures such as bilayers, vesicles, and micelles.[2] In modern cells, vesicles are involved in metabolism, transport, buoyancy control,[21] and enzyme storage. They can also act as natural chemical reaction chambers. A typical vesicle or micelle in aqueous solution forms an aggregate with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single-tail regions in the micelle centre. This phase is caused by the packing behavior of single-tail lipids in a bilayer. Although the protocellular self-assembly process that spontaneously form lipid monolayer vesicles and micelles in nature resemble the kinds of primordial vesicles or protocells that might have existed at the beginning of evolution, they are not as sophisticated as the bilayer membranes of today's living organisms.[22]
Rather than being made up of phospholipids, however, early membranes may have formed from monolayers or bilayers of fatty acids, which may have formed more readily in a prebiotic environment.[23] Fatty acids have been synthesized in laboratories under a variety of prebiotic conditions and have been found on meteorites, suggesting their natural synthesis in nature.[4]
Oleic acid vesicles represent good models of membrane protocells that could have existed in prebiotic times.[24]
Electrostatic interactions induced by short, positively charged, hydrophobic peptides containing 7 amino acids in length or fewer, can attach RNA to a vesicle membrane, the basic cell membrane.[25][26]
Geothermal ponds and clay
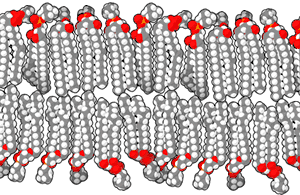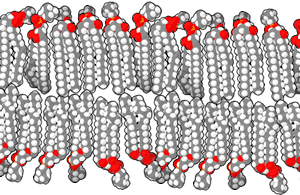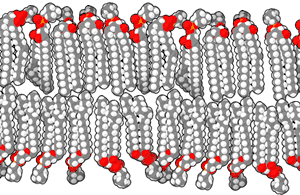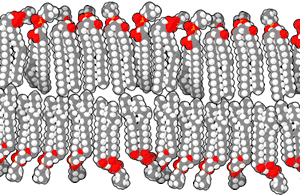
Scientists have suggested that life began in hydrothermal vents in the deep sea, but a 2012 study suggests that inland pools of condensed and cooled geothermal vapor have the ideal characteristics for the origin of life.[27] The conclusion is based mainly on the chemistry of modern cells, where the cytoplasm is rich in potassium, zinc, manganese, and phosphate ions, which are not widespread in marine environments. Such conditions, the researchers argue, are found only where hot hydrothermal fluid brings the ions to the surface—places such as geysers, mud pots, fumaroles and other geothermal features. Within these fuming and bubbling basins, water laden with zinc and manganese ions could have collected, cooled and condensed in shallow pools.[27]
Another study in the 1990s showed that montmorillonite clay can help create RNA chains of as many as 50 nucleotides joined together spontaneously into a single RNA molecule.[5] Later, in 2002, it was discovered that by adding montmorillonite to a solution of fatty acid micelles (lipid spheres), the clay sped up the rate of vesicle formation 100-fold.[5]
Research has shown that some minerals can catalyze the stepwise formation of hydrocarbon tails of fatty acids from hydrogen and carbon monoxide gases—gases that may have been released from hydrothermal vents or geysers. Fatty acids of various lengths are eventually released into the surrounding water,[23] but vesicle formation requires a higher concentration of fatty acids, so it is suggested that protocell formation started at land-bound hydrothermal vents such as geysers, mud pots, fumaroles and other geothermal features where water evaporates and concentrates the solute.[5][28][29]
Montmorillonite bubbles
Another group suggests that primitive cells might have formed inside inorganic clay microcompartments, which can provide an ideal container for the synthesis and compartmentalization of complex organic molecules.[30] Clay-armored bubbles form naturally when particles of montmorillonite clay collect on the outer surface of air bubbles under water. This creates a semi permeable vesicle from materials that are readily available in the environment. The authors remark that montmorillonite is known to serve as a chemical catalyst, encouraging lipids to form membranes and single nucleotides to join into strands of RNA. Primitive reproduction can be envisioned when the clay bubbles burst, releasing the lipid membrane-bound product into the surrounding medium.[30]
Membraneless droplets
Another way to form primitive compartments that may lead to the formation of a protocell is polyesters membraneless structures that have the ability to host biochemicals (proteins and RNA) and/or scaffold the assemblies of lipids around them.[31][32] While these droplets are leaky towards genetic materials, this leakiness could have facilitated the progenote hypothesis.[33]
Membrane transport
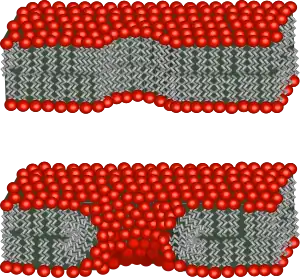
For cellular organisms, the transport of specific molecules across compartmentalizing membrane barriers is essential in order to exchange content with their environment and with other individuals. For example, content exchange between individuals enables horizontal gene transfer, an important factor in the evolution of cellular life.[34] While modern cells can rely on complicated protein machineries to catalyze these crucial processes, protocells must have accomplished this using more simple mechanisms.
Protocells composed of fatty acids[35] would have been able to easily exchange small molecules and ions with their environment.[1] Membranes consisting of fatty acids have a relatively high permeability to molecules such as nucleoside monophosphate (NMP), nucleoside diphosphate (NDP), and nucleoside triphosphate (NTP), and may withstand millimolar concentrations of Mg2+.[36] Osmotic pressure can also play a significant role regarding this passive membrane transport.[1]
Environmental effects have been suggested to trigger conditions under which a transport of larger molecules, such as DNA and RNA, across the membranes of protocells is possible. For example, it has been proposed that electroporation resulting from lightning strikes could enable such transport.[37] Electroporation is the rapid increase in bilayer permeability induced by the application of a large artificial electric field across the membrane. During electroporation, the lipid molecules in the membrane shift position, opening up a pore (hole) that acts as a conductive pathway through which hydrophobic molecules like nucleic acids can pass the lipid bilayer.[38] A similar transfer of content across protocells and with the surrounding solution can be caused by freezing and subsequent thawing. This could, for instance, occur in an environment in which day and night cycles cause recurrent freezing. Laboratory experiments have shown that such conditions allow an exchange of genetic information between populations of protocells.[39] This can be explained by the fact that membranes are highly permeable at temperatures slightly below their phase transition temperature. If this point is reached during the freeze-thaw cycle, even large and highly charged molecules can temporarily pass the protocell membrane.
Some molecules or particles are too large or too hydrophilic to pass through a lipid bilayer even under these conditions, but can be moved across the membrane through fusion or budding of vesicles,[40] events which have also been observed for freeze-thaw cycles.[41] This may eventually have led to mechanisms that facilitate movement of molecules to the inside of the protocell (endocytosis) or to release its contents into the extracellular space (exocytosis).[40]
Artificial models
Langmuir-Blodgett deposition
Starting with a technique commonly used to deposit molecules on a solid surface, Langmuir–Blodgett deposition, scientists are able to assemble phospholipid membranes of arbitrary complexity layer by layer.[42][43] These artificial phospholipid membranes support functional insertion both of purified and of in situ expressed membrane proteins.[43] The technique could help astrobiologists understand how the first living cells originated.[42]
Jeewanu protocells
Jeewanu protocells are synthetic chemical particles that possess cell-like structure and seem to have some functional living properties.[44] First synthesized in 1963 from simple minerals and basic organics while exposed to sunlight, it is still reported to have some metabolic capabilities, the presence of semipermeable membrane, amino acids, phospholipids, carbohydrates and RNA-like molecules.[44][45] However, the nature and properties of the Jeewanu remains to be clarified.[44][45][46]
In a similar synthesis experiment a frozen mixture of water, methanol, ammonia and carbon monoxide was exposed to ultraviolet (UV) radiation. This combination yielded large amounts of organic material that self-organised to form globules or vesicles when immersed in water.[47] The investigating scientist considered these globules to resemble cell membranes that enclose and concentrate the chemistry of life, separating their interior from the outside world. The globules were between 10 to 40 micrometres (0.00039 to 0.00157 in), or about the size of red blood cells. Remarkably, the globules fluoresced, or glowed, when exposed to UV light. Absorbing UV and converting it into visible light in this way was considered one possible way of providing energy to a primitive cell. If such globules played a role in the origin of life, the fluorescence could have been a precursor to primitive photosynthesis. Such fluorescence also provides the benefit of acting as a sunscreen, diffusing any damage that otherwise would be inflicted by UV radiation. Such a protective function would have been vital for life on the early Earth, since the ozone layer, which blocks out the sun's most destructive UV rays, did not form until after photosynthetic life began to produce oxygen.[48]
Bio-like structures
The synthesis of three kinds of "jeewanu" have been reported; two of them were organic, and the other was inorganic. Other similar inorganic structures have also been produced. The investigating scientist (V. O. Kalinenko) referred to them as "bio-like structures" and "artificial cells". Formed in distilled water (as well as on agar gel) under the influence of an electric field, they lack protein, amino acids, purine or pyrimidine bases, and certain enzyme activities. According to NASA researchers, "presently known scientific principles of biology and biochemistry cannot account for living inorganic units" and "the postulated existence of these living units has not been proved".[46]
Ethics and controversy
Protocell research has created controversy and opposing opinions, including critics of the vague definition of "artificial life".[49] The creation of a basic unit of life is the most pressing ethical concern, although the most widespread worry about protocells is their potential threat to human health and the environment through uncontrolled replication.[50]
See also
- Abiogenesis – Natural process by which life arises from non-living matter
- Artificial cell
- Emergence – Phenomenon in complex systems where interactions produce effects not directly predictable from the subsystems
- Entropy and life
- Last universal ancestor
- Protocell Circus, a film
- Pseudo-panspermia
- RNA world hypothesis
- Synthetic biology – Interdisciplinary branch of biology and engineering
References
- Chen, Irene A.; Walde, Peter (July 2010). "From Self-Assembled Vesicles to Protocells". Cold Spring Harb Perspect Biol. 2 (7): a002170. doi:10.1101/cshperspect.a002170. PMC 2890201. PMID 20519344.
- Garwood, Russell J. (2012). "Patterns In Palaeontology: The first 3 billion years of evolution". Palaeontology Online. 2 (11): 1–14. Retrieved June 25, 2015.
- National Science Foundation (2013). "Exploring Life's Origins – Protocells". Retrieved 2014-03-18.
- Chen, Irene A. (8 December 2006). "The Emergence of Cells During the Origin of Life". Science. 314 (5805): 1558–59. doi:10.1126/science.1137541. PMID 17158315.
- Zimmer, Carl (26 June 2004). "What Came Before DNA?". Discover Magazine: 1–5.
- Rasmussen, Steen (2 July 2014). "Scientists Create Possible Precursor to Life". A Letters Journal Exploring the Frontiers of Physics. 107 (2). Astrobiology Web. Retrieved 2014-10-24.
- Alberts, Bruce; Johnson, Alexander; Lewis, Julian; Morgan, David; Raff, Martin; Roberts, Keith; Walter, Peter (2014). Molecular Biology of the Cell (6 ed.). New York: Garland Science. ISBN 978-1317563754. Retrieved 2018-06-15.
- Deamer, D.W.; Dworkin, J.P. (2005). "Chemistry and Physics of Primitive Membranes". Top. Curr. Chem. Topics in Current Chemistry. 259: 1–27. doi:10.1007/b136806. ISBN 3-540-27759-5.
- Walde, P (2006). "Surfactant Assemblies and their various possible roles for the origin(s) of life". Orig. Life Evol. Biosph. 36 (2): 109–50. Bibcode:2006OLEB...36..109W. doi:10.1007/s11084-005-9004-3. hdl:20.500.11850/24036. PMID 16642266. S2CID 8928298.
- Sakuma, Yuka; Imai, Masayuki (2015). "From Vesicles to Protocells: The Roles of Amphiphilic Molecules". Life. 5 (1): 651–75. doi:10.3390/life5010651. PMC 4390873. PMID 25738256.
- Marshall, Michael (14 December 2020). "He may have found the key to the origins of life. So why have so few heard of him? - Hungarian biologist Tibor Gánti is an obscure figure. Now, more than a decade after his death, his ideas about how life began are finally coming to fruition". National Geographic Society. Retrieved 15 December 2020.
- Hugues Bersini (2011). "Minimal cell: the computer scientist's point of view". In Muriel Gargaud; Purificación López-Garcìa; Hervé Martin (eds.). Origins and Evolution of Life: An Astrobiological Perspective. Cambridge University Press. pp. 60–61. ISBN 9781139494595.
- Van Segbroeck S, Nowé A, Lenaerts T (2009). "Stochastic simulation of the chemoton". Artif Life. 15 (2): 213–226. CiteSeerX 10.1.1.398.8949. doi:10.1162/artl.2009.15.2.15203. PMID 19199383. S2CID 10634307.
- Hoenigsberg HF (2007). "From geochemistry and biochemistry to prebiotic evolution...we necessarily enter into Gánti's fluid automata". Genet Mol Res. 6 (2): 358–373. PMID 17624859.
- Shapiro, Robert (12 February 2007). "A Simpler Origin for Life". Scientific American. 296 (6): 46–53. Bibcode:2007SciAm.296f..46S. doi:10.1038/scientificamerican0607-46. PMID 17663224.
- Vodopich, Darrell S.; Moore., Randy (2002). "The Importance of Membranes". Biology Laboratory Manual, 6/a. McGraw-Hill. Retrieved 2014-03-17.
- Chang, Thomas Ming Swi (2007). Artificial cells : biotechnology, nanomedicine, regenerative medicine, blood substitutes, bioencapsulation, cell/stem cell therapy. Hackensack, NJ: World Scientific. ISBN 978-981-270-576-1.
- Knowles, JR (1980). "Enzyme-catalyzed phosphoryl transfer reactions". Annu. Rev. Biochem. 49: 877–919. doi:10.1146/annurev.bi.49.070180.004305. PMID 6250450.
- Campbell, Neil A.; Williamson, Brad; Heyden, Robin J. (2006). Biology: Exploring Life. Boston, MA: Pearson Prentice Hall. ISBN 978-0-13-250882-7.
- Clavin, Whitney (13 March 2014). "How Did Life Arise? Fuel Cells May Have Answers". NASA.
- Walsby, AE (1994). "Gas vesicles". Microbiological Reviews. 58 (1): 94–144. doi:10.1128/MMBR.58.1.94-144.1994. PMC 372955. PMID 8177173.
- Szostak, Jack W. (3 September 2004). "Battle of the Bubbles May Have Sparked Evolution". Howard Hughes Medical Institute.
- National Science Foundation (2013). "Membrane Lipids of Past and Present". Exploring Life's Origins Project – A timeline of Life's Evolution. Retrieved 2014-03-17.
- Douliez, Jean-Paul; Zhendre, Vanessa; Grélard, Axelle; Dufourc, Erick J. (24 November 2014). "Aminosilane/Oleic Acid Vesicles as Model Membranes of Protocells". Langmuir. 30 (49): 14717–24. doi:10.1021/la503908z. PMID 25420203.
- "Peptide glue may have held first protocell components together".
- Kamat, Neha P.; Tobé, Sylvia; Hill, Ian T.; Szostak, Jack W. (2015). "Electrostatic Localization of RNA to Protocell Membranes by Cationic Hydrophobic Peptides". Angewandte Chemie International Edition. 54 (40): 11735–39. doi:10.1002/anie.201505742. PMC 4600236. PMID 26223820.
- Switek, Brian (13 February 2012). "Debate bubbles over the origin of life". Nature –!News.
- Szostak, Jack W. (4 June 2008). "Researchers Build Model Protocell Capable of Copying DNA". HHMI News. Howard Hughes Medical Institute.
- Cohen, Philip (23 October 2003). "Clay's matchmaking could have sparked life". New Scientist.
Journal reference: Science (vol 302, p 618 )
- Stone, Howard A. (7 February 2011). "Clay-armored bubbles may have formed first protocells". Harvard School of Engineering and Applied Sciences.
- Jia, Tony Z.; Chandru, Kuhan; Hongo, Yayoi; Afrin, Rehana; Usui, Tomohiro; Myojo, Kunihiro; Cleaves, H. James (22 July 2019). "Membraneless polyester microdroplets as primordial compartments at the origins of life". Proceedings of the National Academy of Sciences. 116 (32): 15830–35. doi:10.1073/pnas.1902336116. PMC 6690027. PMID 31332006.
- Tokyo Institute of Technology (23 July 2019). "ELSI scientists discover new chemistry that may help explain the origins of cellular life – Chemists find simplest organic molecules can self-assemble to give cell-like structures under early Earth conditions". EurekAlert!. Retrieved 23 July 2019.
- Woese, Carl R.; Fox, George E. (March 1977). "The concept of cellular evolution". Journal of Molecular Evolution. 10 (1): 1–6. Bibcode:1977JMolE..10....1W. doi:10.1007/BF01796132. PMID 903983. S2CID 24613906.
- Gyles, C.; Boerlin, P. (2013-12-06). "Horizontally Transferred Genetic Elements and Their Role in Pathogenesis of Bacterial Disease". Veterinary Pathology. 51 (2): 328–40. doi:10.1177/0300985813511131. ISSN 0300-9858. PMID 24318976. S2CID 206510894.
- Müller, A. W. (June 2006). "Re-creating an RNA world". Cell Mol Life Sci. 63 (11): 1278–93. doi:10.1007/s00018-006-6047-1. PMID 16649141. S2CID 36021694.
- Ma, Wentao; Yu, Chunwu; Zhang, Wentao; Hu., Jiming (Nov 2007). "Nucleotide synthetase ribozymes may have emerged first in the RNA world". RNA. 13 (11): 2012–19. doi:10.1261/rna.658507. PMC 2040096. PMID 17878321.
- Demanèche, S; Bertolla, F; Buret, F; et al. (August 2001). "Laboratory-scale evidence for lightning-mediated gene transfer in soil". Appl. Environ. Microbiol. 67 (8): 3440–44. doi:10.1128/AEM.67.8.3440-3444.2001. PMC 93040. PMID 11472916.
- Neumann, E; Schaefer-Ridder, M; Wang, Y; Hofschneider, PH (1982). "Gene transfer into mouse lyoma cells by electroporation in high electric fields". EMBO J. 1 (7): 841–45. doi:10.1002/j.1460-2075.1982.tb01257.x. PMC 553119. PMID 6329708.
- Litschel, Thomas; Ganzinger, Kristina A.; Movinkel, Torgeir; Heymann, Michael; Robinson, Tom; Hannes Mutschler; Schwille, Petra (2018). "Freeze-thaw cycles induce content exchange between cell-sized lipid vesicles". New Journal of Physics. 20 (5): 055008. Bibcode:2018NJPh...20e5008L. doi:10.1088/1367-2630/aabb96. ISSN 1367-2630.
- Norris, V.; Raine, D.J. (October 1998). "A fission-fussion origin for life". Orig Life Evol Biosph. 28 (4): 523–37. doi:10.1023/A:1006568226145. PMID 9742727. S2CID 24682163.
- Tsuji, Gakushi; Fujii, Satoshi; Sunami, Takeshi; Yomo, Tetsuya (2016-01-19). "Sustainable proliferation of liposomes compatible with inner RNA replication". Proceedings of the National Academy of Sciences. 113 (3): 590–95. Bibcode:2016PNAS..113..590T. doi:10.1073/pnas.1516893113. ISSN 0027-8424. PMC 4725462. PMID 26711996.
- "Scientists Create Artificial Cell Membranes". Astrobiology Magazine. 4 October 2014. Retrieved 2014-05-07.
- Matosevic, Sandro; Paegel, Brian M. (29 September 2013). "Layer-by-layer cell membrane assembly". Nature Chemistry. 5 (11): 958–63. Bibcode:2013NatCh...5..958M. doi:10.1038/nchem.1765. PMC 4003896. PMID 24153375.
- Grote, M (September 2011). "Jeewanu, or the 'particles of life'" (PDF). Journal of Biosciences. 36 (4): 563–70. doi:10.1007/s12038-011-9087-0. PMID 21857103. S2CID 19551399. Archived from the original (PDF) on 2014-03-23.
- Gupta, V. K.; Rai, R. K. (2013). "Histochemical localisation of RNA-like material in photochemically formed self-sustaining, abiogenic supramolecular assemblies 'Jeewanu'". Int. Res. J. Of Science & Engineering. 1 (1): 1–4. ISSN 2322-0015.
- Caren, Linda D.; Ponnamperuma, Cyril (1967). "A review of some experiments on the synthesis of 'Jeewanu'" (PDF). NASA Technical Memorandum X-1439.
- Dworkin, Jason P.; Deamer, David W.; Sandford, Scott A.; Allamandola, Louis J. (30 January 2001). "Self-assembling amphiphilic molecules: Synthesis in simulated interstellar/precometary ices". Proceedings of the National Academy of Sciences of the United States of America. 98 (3): 815–19. Bibcode:2001PNAS...98..815D. doi:10.1073/pnas.98.3.815. PMC 14665. PMID 11158552.
- Mullen, L (5 September 2005). "Building Life from Star-Stuff". Astrobiology Magazine.
- Bedau, M.; Church, G.; Rasmussen, S.; Caplan, A.; Benner, S.; Fussenegger, M.; Collins, J.; Deamer, D. (27 May 2010). "Life after the synthetic cell". Nature. 465 (7297): 422–24. Bibcode:2010Natur.465..422.. doi:10.1038/465422a. PMID 20495545. S2CID 27471255.
- Bedau, Mark A.; Parke, Emily C. (2009). The ethics of protocells moral and social implications of creating life in the laboratory (Online ed.). Cambridge, MA: MIT Press. ISBN 978-0-262-51269-5.
External links
- "Protocells: Bridging Nonliving and Living Matter." Edited by Steen Rasmussen, Mark A. Bedau, Liaochai Chen, David Deamer, David Krakauer, Norman, H.Packard and Peter F. Stadler. MIT Press, Cambridge, Massachusetts. 2008.
- "Living Chemistry & A Natural History of Protocells." Synth-ethic: Art and Synthetic Biology Exhibition (2013) at the Natural History Museum, Vienna, Austria.
- Kenyon, DH; Nissenbaum, A (Apr 1976). "Melanoidin and aldocyanoin microspheres: implications for chemical evolution and early precambrian micropaleontology". J. Mol. Evol. 7 (3): 245–51. Bibcode:1976JMolE...7..245K. doi:10.1007/bf01731491. PMID 778393. S2CID 2995886.