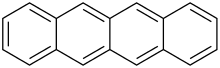
Tetracene
Tetracene, also called naphthacene, is a polycyclic aromatic hydrocarbon. It has the appearance of a pale orange powder. Tetracene is the four-ringed member of the series of acenes. Tetracene is a molecular organic semiconductor, used in organic field-effect transistors (OFETs) and organic light-emitting diodes (OLEDs). In May 2007, researchers from two Japanese universities, Tohoku University in Sendai and Osaka University, reported an ambipolar light-emitting transistor made of a single tetracene crystal.[1] Ambipolar means that the electric charge is transported by both positively charged holes and negatively charged electrons. Tetracene can be also used as a gain medium in dye lasers as a sensitiser in chemoluminescence.
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Tetracene | |
| Other names
Naphthacene Benz[b]anthracene 2,3-Benzanthracene Tetracyclo[8.8.0.03,8.012,17]octadeca-1,3,5,7,9,11,13,15,17-nonaene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.945 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H12 | |
| Molar mass | 228.29 g/mol |
| Appearance | Yellow to orange solid |
| Melting point | 357 °C (675 °F; 630 K) |
| Boiling point | 436.7 |
| Insoluble | |
| -168.0·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Jan Hendrik Schön during his time at Bell Labs (1997–2002) claimed to have developed an electrically pumped laser based on tetracene. However, his results could not be reproduced, and this is considered to be a scientific fraud.[2]
See also
- Tetraphene, also known as benz[a]anthracene
Notes
- Daniel Oberhaus, New Designs Could Boost Solar Cells Beyond Their Limits, Wired, July 11th 2019
References
- T. Takahashi; T. Takenobu; J. Takeya; Y. Iwasa (2007). "Ambipolar Light-Emitting Transistors of a Tetracene Single Crystal". Advanced Functional Materials. 17 (10): 1623–1628. doi:10.1002/adfm.200700046. Archived from the original on 2012-12-10.
- Agin, Dan (2007). Junk Science: An Overdue Indictment of Government, Industry, and Faith Groups That Twist Science for Their Own Gain. Macmillan. ISBN 978-0-312-37480-8.