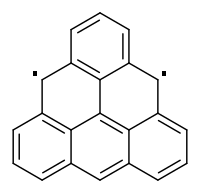
Triangulene
Triangulene (also known as Clar's Hydrocarbon) is the smallest triplet-ground-state polybenzenoid.[1] It exists as a biradical with the chemical formula C
22H
12.[2] It was first hypothesized by Czech chemist Erich Clar in 1953.[3] Its first confirmed synthesis was published in a February 2017 issue of Nature Nanotechnology, in a project led by researchers David Fox and Anish Mistry at the University of Warwick in collaboration with IBM.[4] Other attempts by Japanese researchers have been successful only in making substituted triangulene derivatives.[5]
 | |
| Names | |
|---|---|
| IUPAC name
4H,8H-Dibenzo[cd,mn]pyren-4,8-diyl | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C22H12 | |
| Molar mass | 276.338 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
| Look up triangulene in Wiktionary, the free dictionary. |
| Wikimedia Commons has media related to Triangulene. |
A six-step synthesis yielded two isomers of dihydrotriangulene which were then deposited on xenon or copper base. The researchers used a combined scanning tunneling and atomic force microscope (STM/AFM) to remove individual hydrogen atoms. The synthesized molecule of triangulene remained stable at high-vacuum low-temperature conditions for four days, giving the scientists plenty of time to characterize it (also using STM/AFM).[6]
[n]Triangulenes
Triangulenes, as defined in the previous paragraph, are one case of the wider class of [n]Triangulenes, where n = 1,2,3 etc. is the number of hexagons along an edge of the molecule. Thus, diradical triangulenes may be referred to as [3]triangulenes.
Theory
Hückel (or tight-binding) description of the molecular orbitals of [n]triangulenes predicts[7] that [n]Triangulenes have (n-1) unpaired electrons, associated to (n-1) non-bonding zero mode states. When electron-electron interactions are included, theory predicts[7][8][9] that the spin S of the ground state of [n]triangulenes is 2S=n-1. Thus, [3]triangulenes are predicted to have a S=1 ground state. The intra-molecular exchange interaction, that determines the energy difference between the S=1 ground state and the S=0 excited states, is predicted to be maximal[10] among all Polycyclic Aromatic Hydrocarbons (PAH) diradicals, on account of the maximal overlap of the wave function of the unpaired spin electrons.
The spin of the ground state of [n]triangulenes can be rationalized[7] in terms of a theorem[11] by Elliot H. Lieb, that relates the spin of the ground state of the Hubbard model, at half filling, for a bipartite lattice with a different number of sites of the two sublattices.
Experiments
Thus far, the ultra-high vacuum on-surface syntheses of [n]triangulenes with n = 3,[4] 4,[12] 5[13] and, most recently, 7[14] (the hitherto largest triangulene homologue) have been reported. In addition, the on-surface synthesis of [3]triangulene dimers[7] has also been reported, where inelastic electron tunneling spectroscopy provides a direct evidence of a strong antiferromagnetic coupling between the triangulenes.
References
- "IUPAC Gold Book - biradical". goldbook.iupac.org. Retrieved 2017-02-19.
- "triangulene | C22H12 | ChemSpider". www.chemspider.com. Retrieved 2017-02-19.
- Ball, Philip (2017-02-16). "Elusive triangulene created by moving atoms one at a time". Nature. 542 (7641): 284–285. doi:10.1038/nature.2017.21462. PMID 28202993.
- Pavliček, Niko; Mistry, Anish; Majzik, Zsolt; Moll, Nikolaj; Meyer, Gerhard; Fox, David J.; Gross, Leo (2017-02-13). "Synthesis and characterization of triangulene" (PDF). Nature Nanotechnology. advance online publication (4): 308–311. Bibcode:2017NatNa..12..308P. doi:10.1038/nnano.2016.305. ISSN 1748-3395. PMID 28192389.
- Morita, Yasushi; Suzuki, Shuichi; Sato, Kazunobu; Takui, Takeji (2011). "Synthetic organic spin chemistry for structurally well-defined open-shell graphene fragments". Nature Chemistry. 3 (3): 197–204. Bibcode:2011NatCh...3..197M. doi:10.1038/nchem.985. PMID 21336324.
- Pavliček, Niko; Mistry, Anish; Majzik, Zsolt; Moll, Nikolaj; Meyer, Gerhard; Fox, David J.; Gross, Leo (2017-02-13). "Synthesis and characterization of triangulene" (PDF). Nature Nanotechnology. advance online publication (4): 308–311. Bibcode:2017NatNa..12..308P. doi:10.1038/nnano.2016.305. ISSN 1748-3395. PMID 28192389.
- Fernández-Rossier, J.; Palacios, J. J. (2007-10-23). "Magnetism in Graphene Nanoislands". Physical Review Letters. 99 (17): 177204. doi:10.1103/PhysRevLett.99.177204. hdl:10045/25254.
- Wang, Wei L.; Meng, Sheng; Kaxiras, Efthimios (2008-01-01). "Graphene NanoFlakes with Large Spin". Nano Letters. 8 (1): 241–245. doi:10.1021/nl072548a. ISSN 1530-6984.
- Güçlü, A. D.; Potasz, P.; Voznyy, O.; Korkusinski, M.; Hawrylak, P. (2009-12-10). "Magnetism and Correlations in Fractionally Filled Degenerate Shells of Graphene Quantum Dots". Physical Review Letters. 103 (24): 246805. arXiv:0907.5431. doi:10.1103/PhysRevLett.103.246805.
- Ortiz, Ricardo; Boto, Roberto A.; García-Martínez, Noel; Sancho-García, Juan C.; Melle-Franco, Manuel; Fernández-Rossier, Joaquı́n (2019-09-11). "Exchange Rules for Diradical π-Conjugated Hydrocarbons". Nano Letters. 19 (9): 5991–5997. arXiv:1906.08544. doi:10.1021/acs.nanolett.9b01773. ISSN 1530-6984.
- Lieb, Elliott H. (1989-03-06). "Two theorems on the Hubbard model". Physical Review Letters. 62 (10): 1201–1204. doi:10.1103/PhysRevLett.62.1201.
- Mishra, Shantanu; Beyer, Doreen; Eimre, Kristjan; Liu, Junzhi; Berger, Reinhard; Gröning, Oliver; Pignedoli, Carlo A.; Müllen, Klaus; Fasel, Roman; Feng, Xinliang; Ruffieux, Pascal (2019-07-10). "Synthesis and Characterization of π-Extended Triangulene". Journal of the American Chemical Society. 141 (27): 10621–10625. doi:10.1021/jacs.9b05319. ISSN 0002-7863.
- Su, Jie; Telychko, Mykola; Hu, Pan; Macam, Gennevieve; Mutombo, Pingo; Zhang, Hejian; Bao, Yang; Cheng, Fang; Huang, Zhi-Quan; Qiu, Zhizhan; Tan, Sherman J. R. (2019-07-01). "Atomically precise bottom-up synthesis of π-extended [5]triangulene". Science Advances. 5 (7): eaav7717. doi:10.1126/sciadv.aav7717. ISSN 2375-2548.
- Mishra, Shantanu; Xu, Kun; Eimre, Kristjan; Komber, Hartmut; Ma, Ji; Pignedoli, Carlo; Fasel, Roman; Feng, Xinliang; Ruffieux, Pascal (2021-01-07). "Synthesis and characterization of [7]triangulene". Nanoscale. Accepted Manuscript. doi:10.1039/D0NR08181G.
