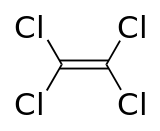
Tetrachloroethylene
Tetrachloroethylene, also known under the systematic name tetrachloroethene, or perchloroethylene, and many other names (and abbreviations such as "perc" or "PERC", and "PCE"), is a chlorocarbon with the formula Cl2C=CCl2. It is a colorless liquid widely used for dry cleaning of fabrics, hence it is sometimes called "dry-cleaning fluid". It has a sweet odor detectable by most people at a concentration of 1 part per million (1 ppm). Worldwide production was about 1 million metric tons (980,000 long tons; 1,100,000 short tons) in 1985.[4]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Tetrachloroethene | |||
| Other names
Perchloroethene; perchloroethylene; perc; PCE | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.388 | ||
| EC Number |
| ||
| KEGG | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1897 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2Cl4 | |||
| Molar mass | 165.82 g/mol | ||
| Appearance | Clear, colorless liquid | ||
| Odor | Mild, chloroform-like[1] | ||
| Density | 1.622 g/cm3 | ||
| Melting point | −19 °C (−2 °F; 254 K) | ||
| Boiling point | 121.1 °C (250.0 °F; 394.2 K) | ||
| 0.15 g/L (25 °C) | |||
| Vapor pressure | 14 mmHg (20 °C)[1] | ||
| −81.6·10−6 cm3/mol | |||
| Viscosity | 0.89 cP at 25 °C | ||
| Hazards | |||
| Main hazards | Harmful (Xn), Dangerous for the environment (N) | ||
| Safety data sheet | See: data page External MSDS | ||
| R-phrases (outdated) | R40 R51/53 | ||
| S-phrases (outdated) | S23 S36/37 S61 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | Not flammable | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration) |
4000 ppm (rat, 4 hr) 5200 ppm (mouse, 4 hr) 4964 ppm (rat, 8 hr)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 100 ppm C 200 ppm (for 5 minutes in any 3-hour period), with a maximum peak of 300 ppm[1] | ||
REL (Recommended) |
Ca Minimize workplace exposure concentrations.[1] | ||
IDLH (Immediate danger) |
Ca [150 ppm][1] | ||
| Related compounds | |||
Related Related organohalides |
Tetrabromoethylene Tetraiodoethylene | ||
Related compounds |
Trichloroethylene Dichloroethene Tetrachloroethane | ||
| Supplementary data page | |||
| Refractive index (n), Dielectric constant (εr), etc. | |||
Thermodynamic data |
Phase behaviour solid–liquid–gas | ||
| UV, IR, NMR, MS | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Production
Michael Faraday first synthesized tetrachloroethylene in 1821 by thermal decomposition of hexachloroethane.
- C2Cl6 → C2Cl4 + Cl2
Most tetrachloroethylene is produced by high temperature chlorinolysis of light hydrocarbons. The method is related to Faraday's discovery since hexachloroethane is generated and thermally decomposes.[4] Side products include carbon tetrachloride, hydrogen chloride, and hexachlorobutadiene.
Several other methods have been developed. When 1,2-dichloroethane is heated to 400 °C with chlorine, tetrachloroethylene is produced by the chemical reaction:
- ClCH2CH2Cl + 3 Cl2 → Cl2C=CCl2 + 4 HCl
This reaction can be catalyzed by a mixture of potassium chloride and aluminium chloride or by activated carbon. Trichloroethylene is a major byproduct, which is separated by distillation.
According to a United States Environmental Protection Agency (EPA) report of 1976, the quantity of tetrachloroethylene produced in the United States in 1973 totaled 320,000 metric tons (706 million lb).[5] By 1993, the volume produced in the United States had dropped to 123,000 metric tons (271 million lb).[6]
Uses
Tetrachloroethylene is an excellent solvent for organic materials. Otherwise it is volatile, highly stable, and nonflammable. For these reasons, it is widely used in dry cleaning. It is also used to degrease metal parts in the automotive and other metalworking industries, usually as a mixture with other chlorocarbons. It appears in a few consumer products including paint strippers and spot removers. It is also used in aerosol preparations.
It is used in neutrino detectors where a neutrino interacts with a neutron in the chlorine atom and converts it to a proton to form argon.
Historical applications
Tetrachloroethylene was once extensively used as an intermediate in the manufacture of HFC-134a and related refrigerants. In the early 20th century, tetrachloroethene was used for the treatment of hookworm infestation.[7]
Health and safety
The acute toxicity of tetrachloroethylene "is moderate to low". "Reports of human injury are uncommon despite its wide usage in dry cleaning and degreasing".[8]
The International Agency for Research on Cancer has classified tetrachloroethylene as a Group 2A carcinogen, which means that it is probably carcinogenic to humans.[9] Like many chlorinated hydrocarbons, tetrachloroethylene is a central nervous system depressant and can enter the body through respiratory or dermal exposure.[10] Tetrachloroethylene dissolves fats from the skin, potentially resulting in skin irritation.
Animal studies and a study of 99 twins showed there is a "lot of circumstantial evidence" that exposure to tetrachloroethylene increases the risk of developing Parkinson's disease ninefold. Larger population studies are planned.[11] Also, tetrachloroethylene has been shown to cause liver tumors in mice and kidney tumors in male rats.[12]
At temperatures over 315 °C (599 °F), such as in welding, tetrachloroethylene can be oxidized into phosgene, an extremely poisonous gas.[13][14]
The U.S. National Institute for Occupational Safety and Health has compiled extensive health and safety information for tetrachloroethylene,[15][16] including recommendations for dry cleaning establishments.[17][18][19][20]
Tetrachloroethylene exposure has been linked to pronounced acquired color vision deficiencies after chronic exposure.[21]
Testing for exposure
Tetrachloroethylene exposure can be evaluated by a breath test, analogous to breath-alcohol measurements. Because it is stored in the body's fat and slowly released into the bloodstream, tetrachloroethylene can be detected in the breath for weeks following a heavy exposure. Tetrachloroethylene and trichloroacetic acid (TCA), a breakdown product of tetrachloroethylene, can be detected in the blood.
In Europe, the Scientific Committee on Occupational Exposure Limits (SCOEL) recommends for tetrachloroethylene an occupational exposure limit (8 hour time-weighted average) of 20 ppm and a short-term exposure limit (15 min) of 40 ppm.[22]
Environmental contamination
Tetrachloroethylene is a common soil contaminant. With a specific gravity greater than 1, tetrachloroethylene will be present as a dense nonaqueous phase liquid (DNAPL) if sufficient quantities are released. Because of its mobility in groundwater, its toxicity at low levels, and its density (which causes it to sink below the water table), cleanup activities are more difficult than for oil spills: oil has a specific gravity less than 1. Recent research on soil and ground water pollution by tetrachloroethylene has focused on in-place remediation. Instead of excavation or extraction for above-ground treatment or disposal, tetrachloroethylene contamination has been successfully remediated by chemical treatment or bioremediation. Bioremediation has been successful under anaerobic conditions by reductive dechlorination by Dehalococcoides sp. and under aerobic conditions by cometabolism by Pseudomonas sp.[23][24] Partial degradation daughter products include trichloroethylene, cis-1,2-dichloroethene and vinyl chloride; full degradation converts tetrachloroethylene to ethene and hydrogen chloride dissolved in water.
Estimates state that 85% of tetrachloroethylene produced is released into the atmosphere; while models from OECD assumed that 90% is released into the air and 10% to water. Based on these models, its distribution in the environment is estimated to be in the air (76.39% - 99.69%), water (0.23% - 23.2%), soil (0.06-7%), with the remainder in the sediment and biota. Estimates of lifetime in the atmosphere vary, but a 1987 survey estimated the lifetime in the air to be about 2 months in the Southern Hemisphere and 5–6 months in the Northern Hemisphere. Degradation products observed in a laboratory include phosgene, trichloroacetyl chloride, hydrogen chloride, carbon dioxide, and carbon monoxide. Tetrachloroethylene is degraded by hydrolysis, and is persistent under aerobic conditions. It is degraded by reductive dechlorination under anaerobic conditions, with degradation products such as trichloroethylene, dichloroethylene, vinyl chloride, ethylene, and ethane.[25] It has an ozone depletion potential of 0.005, where CFC-11 (CCl3F) is 1.
See also
References
- NIOSH Pocket Guide to Chemical Hazards. "#0599". National Institute for Occupational Safety and Health (NIOSH).
- "Compound Summary: Tetrachloroethylene". PubChem. Retrieved 9 September 2020.
- "Tetrachloroethylene". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- M. Rossberg et al. "Chlorinated Hydrocarbons" in Ullmann’s Encyclopedia of Industrial Chemistry, 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_233.pub2
- "Assessment of Hazardous Waste Practices: Organic Chemicals, Pesticides and Explosives Industries" prebpublication issue for EPA Libraries and Solid Waste Management Agencies under contract # 68-01-2919, USEPA 1976
- "Toxicological Profile For Tetrachloroethylene" (PDF). Atlanta, GA: Agency for Toxic Substances and Disease Registry. September 1997. p. 174. Retrieved 2012-09-16. citing C&EN, 1994, Facts and Figures for the Chemical Industry, Chemical and Engineering News, July 4, 1994.
- Young, M.D.; et al. (1960). "The Comparative Efficacy of Bephenium Hydroxynaphthoate and Tetrachloroethylene against Hookworm and other Parasites of Man". American Journal of Tropical Medicine and Hygiene. 9 (5): 488–491. doi:10.4269/ajtmh.1960.9.488. PMID 13787477.
- E.-L. Dreher; T. R. Torkelson; K. K. Beutel (2011). "Chlorethanes and Chloroethylenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o06_o01. ISBN 978-3527306732.
- IARC monograph. Tetrachloroethylene, Vol. 63, p. 159. Last Updated May 20, 1997. Last retrieved June 22, 2007.
- Control of Exposure to Perchloroethylene in Commercial Drycleaning Archived September 1, 2009, at the Wayback Machine. Hazard Controls: Publication 97-157. National Institute for Occupational Safety and Health.
- Industrial Solvent Linked to Increased Risk of Parkinson's Disease Archived March 10, 2010, at the Wayback Machine
- "Solvents: the hazardous chemicals to avoid in everyday life - Meds News". Meds News. Retrieved 2016-01-22.
- "ATSDR - Medical Management Guidelines (MMGs): Tetrachloroethylene (PERC)".
- "OSH Answers:4-Working Safely with Tetrachloroethylene". Government of Canada, Canadian Centre for Occupational Health and Safety. Archived from the original on 2007-07-15. Retrieved 2011-10-13.
- "Tetrachloroethylene (perchloroethylene)". CDC / NIOSH Workplace Safety and Health Topic. October 25, 2010. Retrieved 2016-07-15.
- "Criteria for a Recommended Standard: Occupational Exposure to Tetrachloroethylene (Perchloroethylene) (76-185)". CDC - NIOSH Publications and Products. June 6, 2014. doi:10.26616/NIOSHPUB76128. Retrieved 2016-07-15.
- "Control of Exposure to Perchloroethylene in Commercial Drycleaning (97-154)". CDC - NIOSH Publications and Products. June 6, 2014. doi:10.26616/NIOSHPUB97154. Retrieved 2016-07-15.
- "Control of Exposure to Perchloroethylene in Commercial Drycleaning (Substitution) (97-155)". CDC - NIOSH Publications and Products. June 6, 2014. doi:10.26616/NIOSHPUB97155. Retrieved 2016-07-15.
- "Control of Exposure to Perchloroethylene in Commercial Drycleaning (Machine Design) (97-156)". CDC - NIOSH Publications and Products. June 6, 2014. doi:10.26616/NIOSHPUB97156. Retrieved 2016-07-15.
- "Control of Exposure to Perchloroethylene in Commercial Drycleaning (Ventilation) (97-157)". CDC - NIOSH Publications and Products. June 6, 2014. doi:10.26616/NIOSHPUB97157. Retrieved 2016-07-15.
- Iregren, A (Dec 2002). "Color Vision and Occupational Chemical Exposure: An Overview of Tests and Effects". Neurotoxicity. 23 (6): 719–33. doi:10.1016/S0161-813X(02)00088-8. PMID 12520762.
- "SCOEL recommendations". 2011-04-22. Retrieved 2011-04-22.
- Ryoo, D., Shim, H., Arenghi, F. L. G., Barbieri, P., Wood T. K. (2001). "Tetrachloroethylene, Trichloroethylene, and Chlorinated Phenols Induce Toluene-o-xylene Monooxoygenase Activity in Pseudomonas Stutzeri OX1". Appl Microbiol Biotechnol. 56 (3–4): 545–549. doi:10.1007/s002530100675. PMID 11549035. S2CID 23770815.CS1 maint: multiple names: authors list (link)
- Deckard, L. A., Wills, J. C., Rivers, D. B. (1994). "Evidence for aerobic degradation of tetrachloroethylene by bacterial isolate". Biotechnol. Lett. 16 (11): 1221–1224. doi:10.1007/BF01020855. S2CID 29470943.CS1 maint: multiple names: authors list (link)
- Watts P. (2006). Concise International Chemical Assessment Document 68: TETRACHLOROETHENE, World Health Organization
Further reading
- "Toxicological Profile for Tetrachloroethene". Agency for Toxic Substances and Disease Registry. 1997.
- Doherty, R.E. (2000). "A History of the Production and Use of Carbon Tetrachloride, Tetrachloroethylene, Trichloroethylene and 1,1,1-Trichloroethane in the United States: Part 1 - Historical Background; Carbon Tetrachloride and Tetrachloroethylene". Environmental Forensics. 1 (2): 69–81. doi:10.1006/enfo.2000.0010. S2CID 97680726.
External links
- ATSDR Case Studies in Environmental Medicine: Tetrachloroethylene Toxicity U.S. Department of Health and Human Services
- Tetrachloroethylene (Perchloroethylene) U.S. Department of Health and Human Services
- Australian National Pollutant Inventory (NPI) page
- "Toxic Fumes May Have Made Gunman Snap", by Julian Kesner, New York Daily News, April 20, 2007.
- Sustainable uses and Industry recommendations