Water cluster
In chemistry, a water cluster is a discrete hydrogen bonded assembly or cluster of molecules of water.[1][2] Many such clusters have been predicted by theoretical models (in silico), and some have been detected experimentally, in various contexts such as ice, and bulk liquid water, in the gas phase, in dilute mixtures with non-polar solvents, and as water of hydration in crystal lattices. The simplest example is the water dimer (H2O)2. On the basis of the two state model proposed by Werner Luck it is suggested that the Raman spectrum for water can be explained on the basis of the presence of free and hydrogen bonded hydrogen bonds.[3] The model Luck has application with spectral method Differential Non-equilibrium Energy Spectrum (DNES) by Anton Antonov.[4]
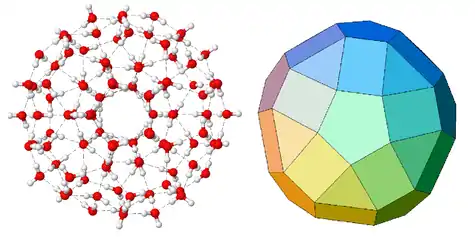
Water clusters have been proposed as an explanation for some anomalous properties of water, such as the unusual variation of density with temperature. Water clusters are also implicated in the stabilization of certain supramolecular structures.[5] They are expected to play a role also in the hydration of molecules and ions dissolved in water.[6][7]
Theoretical predictions
Detailed water models predict the occurrence of water clusters, as configurations of water molecules whose total energy is a local minimum.[8][9][10][11]
Of particular interest are the cyclic clusters (H2O)n; these have been predicted to exist for n = 3 to 60.[12][13] With increasing cluster size the oxygen to oxygen distance is found to decrease which is attributed to so-called cooperative many-body interactions: due to a change in charge distribution the H-acceptor molecule becomes a better H-donor molecule with each expansion of the water assembly. Many isomeric forms seem to exist for the hexamer (H2O)6: from ring, book, bag, cage, to prism shape with nearly identical energy. Two cage-like isomers exist for heptamers (H2O)7, and octamers (H2O)8 are found either cyclic or in the shape of a cube.
Other theoretical studies predict clusters with more complex three-dimensional structures.[14] Examples include the fullerene-like cluster (H2O)28, named the water buckyball, and the 280-water-molecule monster icosahedral network (with each water molecule coordinate to 4 others). The latter, which is 3 nm in diameter, consists of nested icosahedral shells with 280 and 100 molecules.[15][16] There is also an augmented version with another shell of 320 molecules. There is increased stability with the addition of each shell.[17] There are theoretical models of water clusters of more than 700 water molecules,[18][19] but they have not been observed experimentally.
Experimental observations
Experimental study of any supramolecular structures in bulk water is difficult because of their short lifetime: the hydrogen bonds are continually breaking and reforming at the timescales faster than 200 femtoseconds.[20]
Nevertheless, water clusters have been observed in the gas phase and in dilute mixtures of water and non-polar solvents like benzene and liquid helium.[21][22] The detection and characterization of the clusters is achieved with the following methods -far-infrared (FIR),[23] vibration-rotation-tunneling (VRT),[24] EXAFS-spectroscopy and X-Ray,[25] Н-NMR,[26] neutron diffraction,[27] SCC-DFTB Method,[28] DNES.[29] The hexamer is found to have planar geometry in liquid helium, a chair conformation in organic solvents, and a cage structure in the gas phase. Experiments combining IR spectroscopy with mass spectrometry reveal cubic configurations for clusters in the range W8-W10.
When the water is part of a crystal structure as in a hydrate, x-ray diffraction can be used. Conformation of a water heptamer was determined (cyclic twisted nonplanar) using this method.[30] Further, multi-layered water clusters with formulae (H2O)100 trapped inside cavities of several polyoxometalate clusters were also reported by Mueller et. al.[31][32]
Bulk water models
According to the so-called in silico method quantum cluster equilibrium (QCE) theory of liquids W8 clusters dominate the liquid water bulk phase followed by W5 and W6 clusters. In order to facilitate a water triple point the presence of a W24 cluster is invoked. In another model bulk water is built up from a mixture of hexamer and pentamer rings containing cavities capable of enclosing small solutes. In yet another model an equilibrium exists between a cubic water octamer and two cyclic tetramers. However, in spite of much model-making, no model yet has reproduced the experimentally-observed density maximum.[33][34]
The structure of water
A water cluster is composite of two phases, an outer liquid phase and an inner gas phase.[35]
References
- Xantheas, Sotiris; Dunning Jr., Thorn (1993). "Ab initio studies of cyclic water clusters (H2O)n, n=1–6. I. Optimal structures and vibrational spectra". The Journal of Chemical Physics. 99 (11): 8774–8792. doi:10.1063/1.465599.
- Ralf Ludwig (2001). "Water: From Clusters to the Bulk". Angew. Chem. Int. Ed. 40 (10): 1808–1827. doi:10.1002/1521-3773(20010518)40:10<1808::AID-ANIE1808>3.0.CO;2-1. PMID 11385651.
- Luck, Werner; Schiöberg, Dag; Siemann, Ulrich (1980). "Infared Iinvestigation of Water Structure in Desalination MembranesI. Optimal structures and vibrational spectra". J. Chem. Soc., Faraday. 76: 136–147. doi:10.1039/F29807600136.
- Gramatikov, Plamen; Antonov, Anton; Gramatikova, Mari (1992). "A study of the properties and structure variations of water systems under the stimulus of outside influences". Fresenius Journal of Analytical Chemistry. 343 (1): 134–135. doi:10.1007/BF00332070. S2CID 97070621.
- Ghosh, Sujit; Bhardwaj, Parimal (2004). "A Dodecameric Water Cluster Built around a Cyclic Quasiplanar Hexameric Core in an Organic Supramolecular Complex of a Cryptand". Angewandte Chemie. 116 (27): 3661–3664. doi:10.1002/ange.200454002.
- A. D. Kulkarni; S. R. Gadre; S. Nagase (2008). "Quantum chemical and electrostatic studies of anionic water clusters(H2O)n−". Journal of Molecular Structure: THEOCHEM. 851 (1–3): 213. doi:10.1016/j.theochem.2007.11.019.
- A. D. Kulkarni; K. Babu; L. J. Bartolotti; S. R. Gadre. (2004). "Exploring Hydration Patterns of Aldehydes and Amides: Ab Initio Investigations". J. Phys. Chem. A. 108 (13): 2492. Bibcode:2004JPCA..108.2492K. doi:10.1021/jp0368886.
- Fowler, P. W., Quinn, C. M., Redmond, D. B. (1991) Decorated fullerenes and model structures for water clusters, The Journal of Chemical Physics, Vol. 95, No 10, p. 7678.
- Ignatov, I., Mosin, O. V. (2013) Structural Mathematical Models Describing Water Clusters, Journal of Mathematical Theory and Modeling, Vol. 3, No 11, pp. 72-87.
- Keutsch, F. N. and Saykally, R. J. (2001) Water clusters: Untangling the mysteries of the liquid, one molecule at a time, PNAS, Vol. 98, № 19, pp. 10533–10540.
- Maheshwary, Shruti; Patel, Nitin; Sathyamurthy, Narayanasami; Kulkarni, Anand (2001). "Structure and Stability of Water Clusters (H2O)n, n = 8-20". Journal of Physical Chemistry A. 105: 10525–10537. doi:10.1021/jp013141b.
- A. D. Kulkarni; R. K. Pathak; L. J. Bartolotti. (2005). "Structures, Energetics, and Vibrational Spectra of H2O2···(H2O)n, n = 1−6 Clusters: Ab Initio Quantum Chemical Investigations". J. Phys. Chem. A. 109 (20): 4583–90. Bibcode:2005JPCA..109.4583K. doi:10.1021/jp044545h. PMID 16833795.
- S. Maheshwary; N. Patel; N Sathyamurthy; A. D. Kulkarni; S. R. Gadre (2001). "Structure and Stability of Water Clusters (H2O)n, n = 8-20: An Ab Initio Investigation". J. Phys. Chem. A. 105 (46): 10525. Bibcode:2001JPCA..10510525M. doi:10.1021/jp013141b.
- G. S. Fanourgakis; E. Aprà; W. A. de Jong; S. S. Xantheas (2005). "High-level ab initio calculations for the four low-lying families of minima of (H2O)20. II. Spectroscopic signatures of the dodecahedron, fused cubes, face-sharinbucky water g pentagonal prisms, and edge-sharing pentagonal prisms hydrogen bonding networks". J. Chem. Phys. 122 (13): 134304. Bibcode:2005JChPh.122m4304F. doi:10.1063/1.1864892. PMID 15847462.
- Tokmachev, A.M., Tchougreeff, A.L., Dronskowski, R. (2010) Hydrogen-Bond Networks in Water Clusters (H2O)20: An Exhaustive Quantum-Chemical Analysis, ChemPhysChem, Vol. 11, №2, pp. 384–388.
- Sykes, М. (2007) Simulations of RNA Base Pairs in a Nanodroplet Reveal Solvation-Dependent Stability, PNAS, Vol. 104, № 30, pp. 12336–12340.
- Loboda, Oleksandr; Goncharuk, Vladyslav (2010). "Theoretical study on icosahedral water clusters". Chemical Physics Letters. 484 (4–6): 144–147. Bibcode:2010CPL...484..144L. doi:10.1016/j.cplett.2009.11.025.
- Chaplin, M. F. (2013) What is liquid water, Science in Society, Iss. 58, 41-45.
- Zenin, S. V.(2002)Water, Federal Center for Traditional Methods for Diagnostics and Treatment, Moscow
- Smith, Jared D.; Christopher D. Cappa; Kevin R. Wilson; Ronald C. Cohen; Phillip L. Geissler; Richard J. Saykally (2005). "Unified description of temperature-dependent hydrogen-bond rearrangements in liquid water" (PDF). Proc. Natl. Acad. Sci. USA. 102 (40): 14171–14174. Bibcode:2005PNAS..10214171S. doi:10.1073/pnas.0506899102. PMC 1242322. PMID 16179387.
- C. J. Gruenloh; J. R. Carney; C. A. Arrington; T. S. Zwier; S. Y. Fredericks; K. D. Jordan (1997). "Infrared Spectrum of a Molecular Ice Cube: The S4 and D2d Water Octamers in Benzene-(Water)8". Science. 276 (5319): 1678. doi:10.1126/science.276.5319.1678.
- M. R. Viant; J. D. Cruzan; D. D. Lucas; M. G. Brown; K. Liu; R. J. Saykally (1997). "Pseudorotation in Water Trimer Isotopomers Using Terahertz Laser Spectroscopy". J. Phys. Chem. A. 101 (48): 9032. Bibcode:1997JPCA..101.9032V. doi:10.1021/jp970783j.
- Liu, Kun; Fellers, Raymond; Viant, Mark; McLaughlin, Rayan; Brown, Mac; Saykally, Richard (1998). "A long path length pulsed slit valve appropriate for high temperature operation: Infrared spectroscopy of jet‐cooled large water clusters and nucleotide bases". Review of Scientific Instruments. 67 (2).
- Liu, Kun; Cruzan, Jeffery; Saykally, Richard (1996). "Water Clusters". Science. 271 (5251): 929–933. doi:10.1126/science.271.5251.929. S2CID 220091855.
- D Angelo, Paola; Zitolo, Andrea; Aquilanti, Giuliana; Migliorati, Valentina (2013). "Using a Combined Theoretical and Experimental Approach to Understand the Structure and Dynamics of Imidazolium-Based Ionic Liquids/Water Mixtures. 2. EXAFS Spectroscopy". The Journal of Physical Chemistry B. 117 (41): 12516–12524. doi:10.1021/jp404868a.
- Turov, Volodymyr; Krupskaya, Tetiana; Barvinchenko, Valentina; Lipkovska, Natalia; Kartel, Mykola; Suvorova, Liudmyla (2016). "Peculiarities of water cluster formation on the surface of dispersed KCl: The influence of hydrophobic silica and organic media". Colloids and Surfaces A:Physicochemical and Engineering Aspects. 499: 97–102. doi:10.1016/j.colsurfa.2016.03.069.
- Yoshida, Koji; Ishuda, Shigeru; Yamaguchi, Toshio (2019). "Hydrogen bonding and clusters in supercritical methanol–water mixture by neutron diffraction with H/D substitution combined with empirical potential structure refinement modelling". Molecular Physics. 117 (22): 3297–3310. doi:10.1080/00268976.2019.1633481. S2CID 198343685.
- Choi, Tae; Jordan, Kenneth (2010). "Application of the SCC-DFTB Method to H+(H2O)6, H+(H2O)21, and H+(H2O)22". The Journal of Physical Chemistry B. 114 (20): 6932–6936. doi:10.1021/jp912289e. PMID 20433189.
- Todorov, Stefan; Damianova, Anna; Sivriev, Ivan; Antonov, Anton; Galabova, Tatiana (2008). "Water energy spectrum method and investigation of the variations of the H-bond structure of natural waters". Comptes Rendus de l'Académie Bulgare des Sciences. 61 (5251): 857–862.
- M. H. Mir; J. J. Vittal (2007). "Phase Transition Accompanied by Transformation of an Elusive Discrete Cyclic Water Heptamer to a Bicyclic (H2O)7 Cluster". Angew. Chem. Int. Ed. 46 (31): 5925–5928. doi:10.1002/anie.200701779. PMID 17577896.
- T. Mitra; P. Miró; A.-R. Tomsa; A. Merca; H. Bögge; J. B. Ávalos; J. M. Poblet; C. Bo; A. Müller (2009). "Gated and Differently Functionalized (New) Porous Capsules Direct Encapsulates' Structures: Higher and Lower Density Water". Chem. Eur. J. 15 (8): 1844–1852. doi:10.1002/chem.200801602. PMID 19130528.
- A. Müller; E. Krickemeyer; H. Bögge; M. Schmidtmann; S. Roy; A. Berkle (2002). "Changeable Pore Sizes Allowing Effective and Specific Recognition by a Molybdenum-Oxide Based "Nanosponge": En Route to Sphere-Surface and Nanoporous-Cluster Chemistry". Angew. Chem. Int. Ed. 41 (19): 3604–3609. doi:10.1002/1521-3773(20021004)41:19<3604::aid-anie3604>3.0.co;2-t.
- Borowski, Piotr; Jaroniec, Justyna; Janowski, Tomasz; Woliński, Krzysztof (2003). "Quantum cluster equilibrium theory treatment of hydrogen-bonded liquids: Water, methanol and ethanol". Molecular Physics. 101 (10): 1413. Bibcode:2003MolPh.101.1413B. doi:10.1080/0026897031000085083. S2CID 96921359.
- Lehmann, S. B. C.; Spickermann, C.; Kirchner, B. (2009). "Quantum Cluster Equilibrium Theory Applied in Hydrogen Bond Number Studies of Water. 1. Assessment of the Quantum Cluster Equilibrium Model for Liquid Water". Journal of Chemical Theory and Computation. 5 (6): 1640–9. doi:10.1021/ct800310a. PMID 26609856.
- L Shu, L Jegatheesan, V Jegatheesan, CQ Li (2020) The structure of water, Fluid Phase Equilibria 511, 112514
External links
- Water clusters at London South Bank University Link
- The Cambridge Cluster Database - Includes water clusters calculated with various water models and the water clusters explored with ab initio methods.
