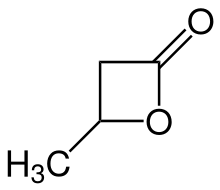
β-Butyrolactone
β-Butyrolactone is the intramolecular carboxylic acid ester (lactone) of the optically active 3-hydroxybutanoic acid. It is produced during chemical synthesis as a racemate. β-Butyrolactone is suitable as a monomer for the production of the biodegradable polyhydroxyalkanoate poly(3-hydroxybutyrate) (PHB). Polymerisation of racemic (RS)-β-butyrolactone provides (RS)-polyhydroxybutyric acid, which, however, is inferior in essential properties (e.g. strength or degradation behaviour) to the (R)-poly-3-hydroxybutyrate originating from natural sources.[3]
 | |
| Names | |
|---|---|
| IUPAC name
4-methyloxetan-2-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.019.392 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
| UN number | 1993 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H6O2 | |
| Molar mass | 86.090 g·mol−1 |
| Appearance | Colourless to light yellow liquid[1] |
| Boiling point | 71–73 °C (160–163 °F; 344–346 K) 39 hPa[2] |
| 268 g·l−1[1] | |
| Solubility | Soluble in various organic solvents[1] |
| Hazards | |
| GHS pictograms |    |
| GHS Signal word | Warning |
| H226, H315, H319, H351 | |
| P201, P202, P210, P233, P240, P241, P242, P243, P264, P280, P281, P302+352, P303+361+353, P305+351+338, P308+313, P321, P332+313, P337+313, P362, P370+378, P403+235, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production
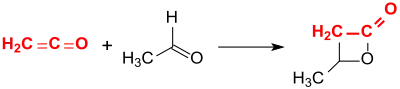
β-Butyrolactone is obtained in 63% yield by the addition of ethanal to ethenone (ketene) in the presence of the clay mineral montmorillonite.[4]
For this purpose, ethenone can also be produced in-situ by dehydrobromination of acetyl bromide with the Hünig base diisopropylethylamine. In the presence of a chiral aluminium complex, the ethenone reacts enantioselectively to (S)-β-butyrolactone in 92% yield with an enantiomeric excess ee of over 98%.[5]
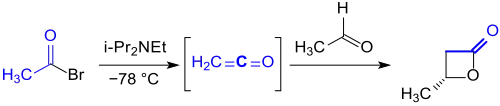
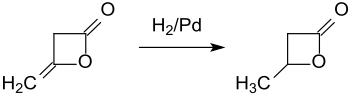
Hydrogenation of diketene at a palladium contact catalyst provides β-butyrolactone in 93% yield.[6]
The asymmetric hydrogenation of diketene with a ruthenium BINAP catalyst to optically active (R)-β-butyrolactone with 97% selectivity and 92% enantiomeric excess is also described.[7]
At 50 °C and approx. 60 bar CO pressure, (R)-2-methyloxirane (propylene oxide) is carbonylated to (R)-β-butyrolactone under retention of the configuration in 95% yield,[8] if a homogeneous carbonylation catalyst [(salph)Al(THF)2][Co(CO)4] according to Geoffrey Coates[9] is used (accessible from a modified aluminium-salene complex [(salph)AlCl and sodium tetracarbonyl cobaltate NaCo(CO)4]).

The carbonylation of 2-methyloxirane in the presence of homogeneous porphyrin-carbonylcobaltate catalysts in tetrahydrofuran also succeeds at approx. 14 bar carbon monoxide partial pressure and yields β-butyrolactone in 97% yield.[10]
Due to the problems with the separation and recycling of homogeneous carbonylation catalysts, heterogeneous polymer analogs have recently also been investigated, which deliver similarly high yields (up to 96%) at 60 bar CO pressure. However, these catalysts do not yet appear to be promising candidates for industrial application as they show drastically lower catalytic activity in 50 mm molar laboratory batches.[11]
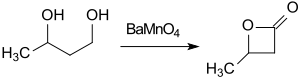
The cheap starting material butane-1,3-diol can be converted with the oxidizing agent barium manganate (BaMnO4) in acetonitrile under microwave irradiation within 1h to β-butyrolactone (74% yield).[12]
Properties
β-Butyrolactone is a clear liquid that smells like acetone or mint.[1] It is miscible with water and soluble in many organic solvents. According to an IARC classification, β-butyrolactone is assigned to group 2B: "possibly carcinogenic".
Use
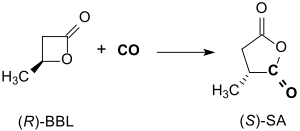
(R)-β-butyrolactone reacts in toluene at approx. 14 bar CO pressure and 55 °C in the presence of a salen complex within 24 h with inversion of the configuration in 94% yield to optically pure (> 99% ee) (S)-methyl succinic anhydride.[13]
Homo- and copolymers from β-butyrolactone
The commercialization of the polyhydroxybutyric acid (PHB) or of homo- and copolymeric polyhydroxyalkanoates as aerobically biodegradable thermoplastics isolated from bacteria under the brand name Biopol of Imperial Chemical Industries (ICI) in 1983 set the starting point for the search for synthetic alternatives which were to avoid the disadvantages of PHB such as brittleness and stiffness, thermal decomposition at temperatures just above the melting temperature (175 - 180 °C) and in particular uncompetitive costs[14] due to expensive fermentation, isolation and purification.
The ring-opening polymerization of (S)-β-butyrolactone with diethylzinc ZnEt2/water produces poly-(S)-3-hydroxybutyrate with ee > 97% under retention of the configuration at the chiral carbon atom:[15]
-beta-BL.svg.png.webp)
With tin compounds (distannoxanes) as catalysts, the polymerization of (R)-β-butyrolactone also produces high molecular weight (Mn > 100,000) synthetic (R)-polyhydroxybutyrates with retention, which resemble the natural polyhydroxyalkanoates.[16]
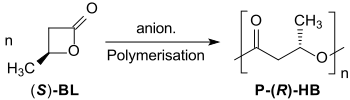
The anionic polymerization of optically active β-butyrolactone leads to crystalline, isotactic polyhydroxybutyrates under inversion, whose low polydispersity Mw/Mn ≈ 1,2 indicate a living polymerization.[17][18]
Also strong bases such as diazabicycloundecene (DBU), 1,5,7-triazabicyclo(4.4.0)dec-5-en (TBD) and the phosphazene BEMP are able to catalyze the ring-opening polymerization of β-butyrolactone in substance at 60 °C achieving a low molecular weight PHBs (Mn < 21.000) with narrow molecular weight distribution.[19]
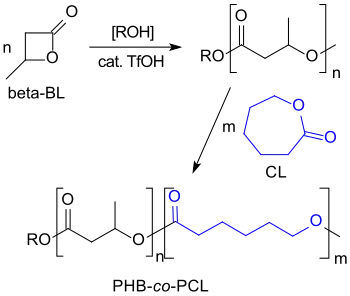
The cationic ring-opening polymerization of β-butyrolactone with strong acids such as trifluoromethanesulfonic acid leads to low-molecular PHBs (Mn < 8,200) with living hydroxyl chain ends to which, for example, caprolactone blocks can be copolymerized.[20]
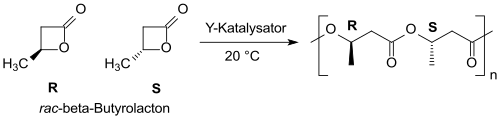
With yttrium-based catalysts racemic β-butyrolactone can be converted into (mainly) syndiotactic PHB with narrow molecular weight distribution.[21][22]
N-heterocyclic carbenes (NHCs) of the imidazol-2-ylidene type are strong nucleophiles and are also suitable as initiators for the ring-opening polymerization of lactones such as β-butyrolactone.[23]

Synthetic PHB variants, which were developed as homopolymers of β-butyrolactone or copolymers with other lactones, have so far not been able to compensate for the weaknesses of the biogenic material - in particular unfavourable mechanical and thermal properties and high price. Instead, new problems with toxic heavy metals in the catalysts (e.g. tin, cobalt or chromium) and atactic polymer components (liquid and difficult to separate) with undesirable material properties have been introduced. Even more than 30 years after its market launch, the economic success of the biopolymer Biopol® and its (bio)synthetic analogues is still modest, and despite ambitious capacity targets (actual global polyhydroxyalkanoate production capacity 2018: approx. 30,000 tons[24]) sales have so far lagged far behind the optimistic forecasts of the manufacturers.
References
- Entry from β-Butyrolactone from TCI Europe, retrieved on 20 December 2018
- Sigma-Aldrich Co., β-Butyrolactone.
- H. Abe; I. Matsubara; Y. Doi; Y. Hori; A. Yamaguchi (1994). "Physical properties and enzymatic degradability of poly(3-hydroxybutyrate) stereoisomers with different stereoregularities". Macromolecules. 27 (21). pp. 6018–6025. doi:10.1021/ma00099a013.
- US 2580714, F.G. Young, J.T. Fitzpatrick, "Production of beta-hydroxy carboxylic acid lactones from ketene and aldehyde with clay catalyst", issued 1952-1-1, assigned to Union Carbide and Carbon Corp.
- S.G. Nelson; W.S. Cheung; A.J. Kassick; M.A. Hilfiker (2002). "A de novo enantioselective total synthesis of (-)-laulimalide". J. Amer. Chem. Soc. 124 (46). pp. 13654–13655. doi:10.1021/ja028019k. PMID 12431077.
- US 2763664, J. Sixt, "Process for manufacturing β-butyrolactone from diketene", issued 1956-9-18, assigned to Wacker-Chemie GmbH
- T. Ohta; T. Miyake; H. Takaya (1992). "An efficient synthesis of optically active 4-methyloxetan-2-one: asymmetric hydrogenation of diketene catalysed by binap–ruthenium(II) complexes [binap = 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl]". J. Chem. Soc., Chem. Commun. 0 (23). pp. 1725–1726. doi:10.1039/C39920001725.
- Y.D.Y.L. Getzler; V. Mahadevan; E.B. Lobkovsky; G.W. Coates (2002). "Synthesis of β-lactones: a highly active and selective catalyst for epoxide carbonylation". J. Amer. Chem. Soc. 124 (7). pp. 1174–1175. doi:10.1021/ja017434u. PMID 11841278.
- "Catalysts for Carbonylation". Aldrich ChemFiles 2007, 7.5, 3. Sigma Aldrich. 2007. Retrieved 2018-12-20.
- US 2012123137, S.D. Allen, R.R. Valente, H. Lee, A.E. Cherian, D.L. Bunning, N.A. Clinton, O.S. Fruchey, B.D. Dombek, "Process for beta-lactone production", issued 2012-5-17, assigned to Novomer, Inc.
- J. Jiang; S. Yoon (2018). "A metalated porous porphyrin polymer with [Co(CO)4]− anion as an efficient heterogeneous catalyst for ring expanding carbonylation". Scientific Reports. 8 (13243). pp. 1–6. doi:10.1038/s41598-018-31475-6. PMC 6125460. PMID 30185794.
- M.C. Bagley; Z. Lin; D.J. Phillips; A.E. Graham (2009). "Barium manganate in microwave-assisted oxidation reactions: synthesis of lactones by oxidative cyclization reactions". Tetrahedron Lett. 50 (49). pp. 6823–6825. doi:10.1016/j.tetlet.2009.09.117.
- Y.D.Y.L. Getzler; V. Kundnani; E.B. Lobkovsky; G.W. Coates (2004). "Catalytic carbonylation of β-lactones to succinic anhydrides". J. Amer. Chem. Soc. 126 (22). pp. 6842–6843. doi:10.1021/ja048946m. PMID 15174834.
- "ICI reduces cost, ups capacity for Biopol". ICIS. 1991-09-22. Retrieved 2018-12-20.
- Y. Zhang; R.A. Gross; R.W. Lenz (1990). "Stereochemistry of the ring-opening polymerization of (S)-β-butyrolactone". Macromolecules. 23 (13). pp. 3206–3212. doi:10.1021/ma00215a002.
- Y. Hori; M. Suzuki; A. Yamaguchi; T. Nishishita (1993). "Ring-opening polymerization of optically active β-butyrolactone using distannoxane catalysts: Synthesis of high molecular weight poly(3-hydroxybutyrate)". Macromolecules. 26 (20). pp. 5533–5534. doi:10.1021/ma00072a037.
- Z. Jedlinski; P. Kurcak (1998). "First facile synthesis of biomimetic poly (R)-3-hydroxybutyrate via regioselective anionic polymerization of (S)-β-butyrolactone". Macromolecules. 31 (19). pp. 6718–6720. doi:10.1021/ma980663p.
- R. Kurcak; M. Smiga; Z. Jedlinski (2002). "β-Butyrolactone polymerization initiated with tetrabutylammonium carboxylates: a novel approach to biomimetic polyester synthesis". J. Polym. Sci., Part A: Polym. Chem. 40 (13). pp. 2184–2189. doi:10.1002/pola.10285.
- C.G. Jaffredo; J.-F. Carpentier; S.M. Guillaume (2012). "Controlled ROP of β-butyrolactone simply mediated by amidine, guanidine, and phophazene organocatalysts". Macromol. Rapid Comun. 33 (22). pp. 1938–1944. doi:10.1002/marc.201200410. PMID 22887774.
- A. Couffin; B. Martin-Vaca; D. Bourissou; C. Navarro (2014). "Selective O-acyl ring-opening of β-butyrolactone catalyzed by trifluoromethane sulfonic acid: application to the preparation of well-defined block copolymers". Polym. Chem. 5 (1). pp. 161–168. doi:10.1039/C3PY00935A.
- A. Amgoune; C.M. Thomas; S. Illinca; T. Roisnel; J.-F. Carpentier (2006). "Highly active, productive, and syndiospecific yttrium initiators for the polymerization of racemic β-butyrolactone". Angew. Chem. Int. Ed. 45 (17). pp. 2782–2784. doi:10.1002/anie.200600058. PMID 16548028.
- J.-F. Carpentier (2010). "Discrete metal catalysts for stereoselective ring-opening polymerization of chiral racemic β-lactones". Macromol. Rapid Commun. 31 (19). pp. 1696–1705. doi:10.1002/marc.201000114. PMID 21567583.
- W.N. Ottou; H. Sardon; D. Mecerryes; J. Vignolle; D. Taton (2016). "Update and challenges in organo-mediated polymerization reactions" (PDF). Progress in Polymer Science. 56. pp. 64–115. doi:10.1016/j.progpolymsci.2015.12.001.
- Bioplastic Markt Daten, abgerufen am 20. Dezember 2018.