Alkali metal oxide
The alkali metals react with oxygen to form several different compounds: suboxides, oxides, peroxides, superoxides, and ozonides. They all react violently with water.

A sample of sodium peroxide.
Alkali metal suboxides
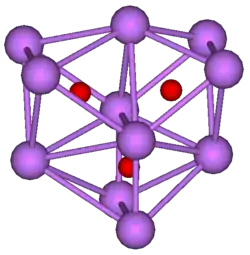
Structure of undecacaesium trioxide.
- Hexarubidium monoxide (Rb6O) h
- Nonarubidium dioxide (Rb9O2)
- Caesium monoxide (CsO)
- Tricaesium monoxide (Cs3O) is a dark green solid.
- Tetracaesium monoxide (Cs4O)
- Heptacaesium monoxide (Cs7O)
- Tricaesium dioxide (Cs3O2)
- Heptacaesium dioxide (Cs7O2)
- Undecacaesium trioxide (Cs11O3)
- Undecacaesium monorubidium trioxide (Cs11RbO3)
- Undecacaesium dirubidium trioxide (Cs11Rb2O3)
- Undecacaesium trirubidium trioxide (Cs11Rb3O3)
Alkali metal oxides
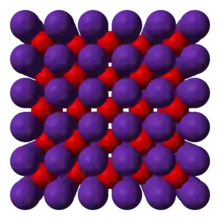
Crystal structure of rubidium oxide.
- Lithium oxide (Li2O) is the lightest alkali metal oxide and a white solid. It melts at 1570 °C.
- Sodium oxide (Na2O) is a white solid that melts at 1132 °C and decomposes at 1950 °C. It is a component of glass.
- Potassium oxide (K2O) is a pale yellow solid that decomposes at 350 °C.
- Rubidium oxide (Rb2O) is a yellow solid that melts at 500 °C.
- Caesium oxide (Cs2O) is a yellow-orange solid that melts at 490 °C.
Alkali metal peroxides
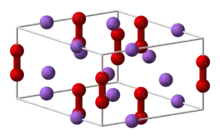
Crystal structure of sodium peroxide.
- Lithium peroxide (Li2O2) is a white solid that melts at 195 °C. It reacts with carbon dioxide to form lithium carbonate and oxygen.
- Sodium peroxide (Na2O2) is a pale yellow solid that melts at 460 °C and decomposes at 657 °C.
- Potassium peroxide (K2O2) is a yellow solid that melts at 490 °C.
- Rubidium peroxide (Rb2O2) is produced when rubidium stands in air.
- Caesium peroxide (Cs2O2) is produced by the decomposition of caesium oxide above 400 °C.
Alkali metal superoxides
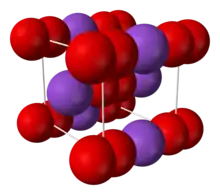
Crystal structure of potassium superoxide.
- Lithium superoxide (LiO2) has only been isolated in matrix isolation at 15 K.
- Sodium superoxide (NaO2) is a yellow-orange solid that melts at 551.7 °C. It is made by the high-pressure oxidation of sodium peroxide.
- Potassium superoxide (KO2) is a yellow solid that decomposes at 560 °C. It is used as a CO2 scrubber, H2O dehumidifier, and O2 generator in rebreathers, spacecraft, submarines, and spacesuit life support systems.
- Rubidium superoxide (RbO2) is produced when rubidium burns in air.
- Caesium superoxide (CsO2) is produced when caesium burns in air.
Alkali metal ozonides
- Lithium ozonide (LiO3) is a red solid which is produced from caesium ozonide via an ion-exchange process.
- Sodium ozonide (NaO3) is a red solid which is produced from caesium ozonide via an ion-exchange process.[1]
- Potassium ozonide (KO3) is a dark red solid which is produced when potassium is burned in ozone or exposed to air for years.
- Rubidium ozonide (RbO3) is a dark red solid which is produced when rubidium is burned in ozone.
- Caesium ozonide (CsO3) is a dark red solid which is produced when caesium is burned in ozone.[2]
References
- Klein, W.; Armbruster, K.; Jansen, M. (1998). "Synthesis and crystal structure determination of sodium ozonide". Chemical Communications (6): 707–708. doi:10.1039/a708570b.
- F. A. Cotton and G. Wilkinson "Advanced Inorganic Chemistry", 5th edition (1988), p.462
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
