Rubidium oxide
Rubidium oxide is the chemical compound with the formula Rb2O. Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. The rubidium content in minerals is often calculated and quoted in terms of Rb2O. In reality, the rubidium is typically present as a component of (actually, an impurity in) silicate or aluminosilicate. A major source of rubidium is lepidolite, KLi2Al(Al,Si)3O10(F,OH)2, wherein Rb sometimes replaces K.
 | |
| Names | |
|---|---|
| IUPAC name
Rubidium oxide | |
| Other names
Rubidium(I) oxide Dirubidium oxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.038.161 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Rb2O | |
| Molar mass | 186.94 g/moL |
| Appearance | Yellow solid |
| Density | 4 g/cm3 |
| Melting point | >500 °C |
| Reacts violently to give RbOH | |
| +1527.0·10−6 cm3/mol | |
| Structure | |
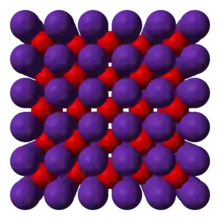
| Antifluorite (cubic), cF12 | |
| Fm3m, No. 225 | |
| Tetrahedral (Rb+); cubic (O2−) | |
| Hazards | |
| Main hazards | Corrosive, reacts violently with water |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other cations |
Lithium oxide Sodium oxide Potassium oxide Caesium oxide |
| Rubidium suboxide Rubidium peroxide Rubidium superoxide | |
Related compounds |
Rubidium hydroxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rb2O is a yellow colored solid. The related species Na2O, K2O, and Cs2O are colorless, pale-yellow, and orange, respectively.
The alkali metal oxides M2O (M = Li, Na, K, Rb) crystallise in the antifluorite structure. In the antifluorite motif the positions of the anions and cations are reversed relative to their positions in CaF2, with rubidium ions 8 coordinate (cubic) and oxide ions 4 coordinate (tetrahedral).[1]
Properties
Like other alkali metal oxides, Rb2O is a strong base. Thus, Rb2O reacts exothermically with water to form rubidium hydroxide.
- Rb2O + H2O → 2 RbOH
So reactive is Rb2O toward water that it is considered hygroscopic. Upon heating, Rb2O reacts with hydrogen to rubidium hydroxide and rubidium hydride:[2]
- Rb2O + H2 → RbOH + RbH
Synthesis
For laboratory use, RbOH is usually used in place of the oxide. RbOH can be purchased for ca. US$5/g (2006). The hydroxide is more useful, less reactive toward atmospheric moisture, and less expensive than the oxide.
As for most alkali metal oxides,[3] the best synthesis of Rb2O does not entail oxidation of the metal but reduction of the anhydrous nitrate:
- 10 Rb + 2 RbNO3 → 6 Rb2O + N2
Typical for alkali metal hydroxides, RbOH cannot be dehydrated to the oxide. Instead, the hydroxide can be decomposed to the oxide (by reduction of the hydrogen ion) using Rb metal:
- 2 Rb + 2 RbOH → 2 Rb2O + H2
Metallic Rb reacts with O2, as indicated by its tendency to rapidly tarnish in air. The tarnishing process is relatively colorful as it proceeds via bronze-colored Rb6O and copper-colored Rb9O2.[4] The suboxides of rubidium that have been characterized by X-ray crystallography include Rb9O2 and Rb6O, as well as the mixed Cs-Rb suboxides Cs11O3Rbn (n = 1, 2, 3).[5]
The final product of oxygenation of Rb is principally RbO2, rubidium superoxide:
- Rb + O2 → RbO2
This superoxide can then be reduced to Rb2O using excess rubidium metal:
- 3 Rb + RbO2 → 2 Rb2O
References
- Wells, Alexander Frank (1984). Structural Inorganic Chemistry (5th ed.). Oxford: Clarendon Press. ISBN 978-0-19-855370-0.
- Nechamkin, Howard (1968). The chemistry of the elements. New York: McGraw-Hill. p. 34.
- Holleman, A.F.; Wiberg, E., eds. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 978-0-12-352651-9.
- Holleman, A.F.; Wiberg, E., eds. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 978-0-12-352651-9.
- Simon, A. (1997). "Group 1 and 2 suboxides and subnitrides — Metals with atomic size holes and tunnels". Coordination Chemistry Reviews. 163: 253–270. doi:10.1016/S0010-8545(97)00013-1.
Further reading
- "Rubidium Oxide". DiracDelta.co.uk science and engineering encyclopedia. Dirac Delta Consultants. Archived from the original on 11 December 2011. Retrieved 16 November 2011.
- "Rubidium compounds: dirubidium oxide". WebElements: the periodic table on the web. WebElements. Retrieved 16 November 2011.
- "Rubidium Oxide". fishersci.com. Thermo Fisher Scientific. Archived from the original on 18 April 2012. Retrieved 16 November 2011.
| Wikimedia Commons has media related to Rubidium oxide. |
