Anthrax lethal factor endopeptidase
Anthrax lethal factor endopeptidase (EC 3.4.24.83, lethal toxin) is an enzyme that catalyzes the hydrolysis of mitogen-activated protein kinase kinases. This enzyme is a component of the lethal factor produced by the bacterium Bacillus anthracis. The preferred cleavage site can be denoted by BBBBxHxH, in which B denotes a basic amino acid Arg or Lys, H denotes a hydrophobic amino acid, and x is any amino acid.[2]
| Anthrax lethal factor endopeptidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
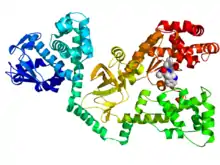 Crystallographic structure of anthrax lethal factor (rainbow colored cartoon, N-terminus = blue, C-terminus = red) complexed with the inhibitor GM6001 (space-filling model, carbon = white, oxygen = red, nitrogen = blue).[1] | |||||||||
| Identifiers | |||||||||
| EC number | 3.4.24.83 | ||||||||
| CAS number | 477950-41-7 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
References
- PDB: 1PWU; Turk BE, Wong TY, Schwarzenbacher R, Jarrell ET, Leppla SH, Collier RJ, Liddington RC, Cantley LC (January 2004). "The structural basis for substrate and inhibitor selectivity of the anthrax lethal factor". Nat. Struct. Mol. Biol. 11 (1): 60–6. doi:10.1038/nsmb708. PMID 14718924.
- Pannifer AD, Wong TY, Schwarzenbacher R, Renatus M, Petosa C, Bienkowska J, Lacy DB, Collier RJ, Park S, Leppla SH, Hanna P, Liddington RC (November 2001). "Crystal structure of the anthrax lethal factor" (PDF). Nature. 414 (6860): 229–33. doi:10.1038/n35101998. PMID 11700563.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.