Neprilysin
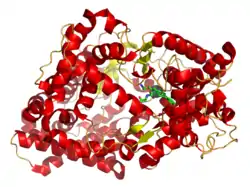
Neprilysin (/ˌnɛprɪˈlaɪsɪn/), also known as membrane metallo-endopeptidase (MME), neutral endopeptidase (NEP), cluster of differentiation 10 (CD10), and common acute lymphoblastic leukemia antigen (CALLA) is an enzyme that in humans is encoded by the MME gene. Neprilysin is a zinc-dependent metalloprotease that cleaves peptides at the amino side of hydrophobic residues and inactivates several peptide hormones including glucagon, enkephalins, substance P, neurotensin, oxytocin, and bradykinin.[5] It also degrades the amyloid beta peptide whose abnormal folding and aggregation in neural tissue has been implicated as a cause of Alzheimer's disease. Synthesized as a membrane-bound protein, the neprilysin ectodomain is released into the extracellular domain after it has been transported from the Golgi apparatus to the cell surface.
Neprilysin is expressed in a wide variety of tissues and is particularly abundant in kidney. It is also a common acute lymphocytic leukemia antigen that is an important cell surface marker in the diagnosis of human acute lymphocytic leukemia (ALL). This protein is present on leukemic cells of pre-B phenotype, which represent 85% of cases of ALL.[5]
Hematopoietic progenitors expressing CD10 are considered "common lymphoid progenitors", which means they can differentiate into T, B or natural killer cells.[6] CD10 is of use in hematological diagnosis since it is expressed by early B, pro-B and pre-B lymphocytes, and by lymph node germinal centers.[7] Hematologic diseases in which it is positive include ALL, angioimmunoblastic T cell lymphoma, Burkitt lymphoma, chronic myelogenous leukemia in blast crisis (90%), diffuse large B-cell lymphoma (variable), follicular center cells (70%), hairy cell leukemia (10%), and myeloma (some). It tends to be negative in acute myeloid leukemia, chronic lymphocytic leukemia, mantle cell lymphoma, and marginal zone lymphoma. CD10 is found on non-T ALL cells, which derive from pre-B lymphocytes, and in germinal center-related non-Hodgkin lymphoma such as Burkitt lymphoma and follicular lymphoma, but not on leukemia cells or lymphomas, which originate in more mature B cells.[8]
Amyloid beta regulation
Neprilysin-deficient knockout mice show both Alzheimer's-like behavioral impairment and amyloid-beta deposition in the brain,[9] providing strong evidence for the protein's association with the Alzheimer's disease process. Because neprilysin is thought to be the rate-limiting step in amyloid beta degradation,[10] it has been considered a potential therapeutic target; compounds such as the peptide hormone somatostatin have been identified that increase the enzyme's activity level.[11] Declining neprilysin activity with increasing age may also be explained by oxidative damage, known to be a causative factor in Alzheimer's disease; higher levels of inappropriately oxidized neprilysin have been found in Alzheimer's patients compared to cognitively normal elderly people.[12]
Signaling peptides
Neprilysin is also associated with other biochemical processes, and is particularly highly expressed in kidney and lung tissues. Inhibitors have been designed with the aim of developing analgesic and antihypertensive agents that act by preventing neprilysin's activity against signaling peptides such as enkephalins, substance P, endothelin, and atrial natriuretic peptide.[13][14]
Associations have been observed between neprilysin expression and various types of cancer; however, the relationship between neprilysin expression and carcinogenesis remains obscure. In cancer biomarker studies, the neprilysin gene is often referred to as CD10 or CALLA. In some types of cancer, such as metastatic carcinoma and some advanced melanomas, neprilysin is overexpressed;[15] in other types, most notably lung cancers, neprilysin is downregulated, and thus unable to modulate the pro-growth autocrine signaling of cancer cells via secreted peptides such as mammalian homologs related to bombesin.[16] Some plant extracts (methanol extracts of Ceropegia rupicola, Kniphofia sumarae, Plectranthus cf barbatus, and an aqueous extract of Pavetta longiflora) were found able to inhibit the enzymatic activity of neutral endopeptidase.[17]
Inhibitors
Inhibitors have been designed with the aim of developing analgesic and antihypertensive agents that act by preventing neprilysin's activity against signaling peptides such as enkephalins, substance P, endothelin, and atrial natriuretic peptide.[13][14]
Some are intended to treat heart failure.[18]
- Sacubitril/valsartan (Entresto/LCZ696), which has been tested against enalapril in patients with heart failure.[18]
- Sacubitril (AHU-377), a prodrug which is a component of sacubitril/valsartan
- Sacubitrilat (LBQ657), the active form of sacubitril
- RB-101, an enkephalinase inhibitor, used in scientific research.
- UK-414,495
- Omapatrilat (dual inhibitor of NEP and angiotensin-converting enzyme) developed by BMS did not receive FDA approval due to angioedema safety concerns.
- Ecadotril
- Candoxatril
Other dual inhibitors of NEP with ACE/angiotensin receptor are (in 2003) being developed by pharmaceutical companies.[19]
Immunochemistry
CD10 is used in clinical pathology for diagnostic purpose.
In lymphomas and leukemias
- Acute lymphoblastic leukemia (ALL) cells are CD10+.
- Follicular lymphoma (follicle centre cell lymphoma) are CD10+.
- Burkitt Lymphoma cells are CD10+.
- CD10+ diffuse large B cell lymphoma (CD10+ DLBCL)[20]
- Angioimmunoblastic T cell lymphoma (AITL) are CD10+[25][26] and distinguishes AITL from other T cell lymphomas (CD10−)[27]
- Some benign T cells can be CD10+[28]
In epithelial tumors
- Clear cell renal cell carcinoma (Clear cell RCC)
- CD10+ distinguishes renal cell carcinoma, conventional type with eosinophilic morphology from its mimickers. Chromophobe carcinoma and oncocytoma are CD10−.[29]
- Pancreatic tumors
- Solid pseudopapillary tumours are CD10+.[30]
- CD10+ differentiates mucinous cystic neoplasms (CD10+/CK20+) from intraductal papillary mucinous neoplasm of branch duct type (CD10−/CK20-).[30]
- Cutaneous tumors
- CD10 may differentiate basal cell carcinoma (CD10 epithelial staining) from trichoblastoma (CD10 peritumoral stromal staining), basal cell carcinoma with follicular differentiation (CD10 stromal and epithelial staining)[31] and squamous cell carcinoma (strong stromal staining).[32]
- CD10 differentiates CD10+ atypical fibroxanthoma from CD10− spindle cell melanoma and sarcomatoid squamous cell carcinoma.
- Urothelial tumors express CD10 (42-67%).[33]
- CD10 expression is strongly correlated with high tumor grade and stage in urothelial carcinoma of the bladder. CD10 may be associated with tumor progression in bladder cancer pathogenesis.[34]
In other tumors
- CD10 expression might be one of the characteristics of müllerian system-derived neoplastic mesenchymal cells.[35]
- Normal endometrial stroma[36]
- Endometrial stromal sarcoma (ESS) are CD10+ (Smooth muscle tumors are usually CD10−,[37] but can be CD10+ [35]
- Malignant müllerian mixed tumor (MMMT)
- Müllerian adenosarcoma
- Uterine high-grade leiomyosarcoma
- Uterine rhabdomyosarcoma
- Vascular tumors
- Epithelioid hemangioendothelioma are mostly CD10+.[38]
- Hemangioblastoma is usually CD10− (metastatic renal cell carcinoma is CD10+)[39][40]
References
- GRCh38: Ensembl release 89: ENSG00000196549 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000027820 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Entrez Gene: Membrane metallo-endopeptidase".
- Galy, Anne; Travis, Marilyn; Cen, Dazhi; Chen, Benjamin (Oct 1995). "Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset". Immunity. 3 (4): 459–73. doi:10.1016/1074-7613(95)90175-2. PMID 7584137.
- Singh C (2011-02-25). "CD10". CD Markers. PathologyOutlines.com, Inc.
- Papandreou CN, Nanus DM (January 2010). "Is methylation the key to CD10 loss?". J. Pediatr. Hematol. Oncol. 32 (1): 2–3. doi:10.1097/MPH.0b013e3181c74aca. PMID 20051779.
- Madani R, Poirier R, Wolfer DP, Welzl H, Groscurth P, Lipp HP, Lu B, El Mouedden M, Mercken M, Nitsch RM, Mohajeri MH (December 2006). "Lack of neprilysin suffices to generate murine amyloid-like deposits in the brain and behavioral deficit in vivo". J. Neurosci. Res. 84 (8): 1871–8. doi:10.1002/jnr.21074. PMID 16998901. S2CID 46527377.
- Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ, Hama E, Sekine-Aizawa Y, Saido TC (February 2000). "Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition". Nat. Med. 6 (2): 143–50. doi:10.1038/72237. PMID 10655101. S2CID 22431826.
- Iwata N, Higuchi M, Saido TC (November 2005). "Metabolism of amyloid-beta peptide and Alzheimer's disease". Pharmacol. Ther. 108 (2): 129–48. doi:10.1016/j.pharmthera.2005.03.010. PMID 16112736.
- Wang DS, Iwata N, Hama E, Saido TC, Dickson DW (October 2003). "Oxidized neprilysin in aging and Alzheimer's disease brains". Biochem. Biophys. Res. Commun. 310 (1): 236–41. doi:10.1016/j.bbrc.2003.09.003. PMID 14511676.
- Sahli S, Stump B, Welti T, Schweizer WB, Diederich R, Blum-Kaelin D, Aebi JD, Böhm HJ (April 2005). "A New Class of Inhibitors for the Metalloprotease Neprilysin Based on a Central Imidazole Scaffold". Helvetica Chimica Acta. 88 (4): 707–730. doi:10.1002/hlca.200590050.
- Oefner C, Roques BP, Fournie-Zaluski MC, Dale GE (February 2004). "Structural analysis of neprilysin with various specific and potent inhibitors". Acta Crystallogr. D. 60 (Pt 2): 392–6. doi:10.1107/S0907444903027410. PMID 14747736.
- Velazquez EF, Yancovitz M, Pavlick A, Berman R, Shapiro R, Bogunovic D, O'Neill D, Yu YL, Spira J, Christos PJ, Zhou XK, Mazumdar M, Nanus DM, Liebes L, Bhardwaj N, Polsky D, Osman I (2007). "Clinical relevance of neutral endopeptidase (NEP/CD10) in melanoma". J Transl Med. 5 (1): 2. doi:10.1186/1479-5876-5-2. PMC 1770905. PMID 17207277.
- Cohen AJ, Bunn PA, Franklin W, Magill-Solc C, Hartmann C, Helfrich B, Gilman L, Folkvord J, Helm K, Miller YE (February 1996). "Neutral endopeptidase: variable expression in human lung, inactivation in lung cancer, and modulation of peptide-induced calcium flux". Cancer Res. 56 (4): 831–9. PMID 8631021.
- Alasbahi R, Melzig MF (January 2008). "Screening of some Yemeni medicinal plants for inhibitory activity against peptidases". Pharmazie. 63 (1): 86–8. doi:10.1055/s-2008-1047849. PMID 18271311.
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR (Sep 2014). "Angiotensin-neprilysin inhibition versus enalapril in heart failure". The New England Journal of Medicine. 371 (11): 993–1004. doi:10.1056/NEJMoa1409077. hdl:10993/27659. PMID 25176015.
- Venugopal J (2003). "Pharmacological modulation of the natriuretic peptide system". Expert Opinion on Therapeutic Patents. 13 (9): 1389–1409. doi:10.1517/13543776.13.9.1389. S2CID 85007768.
- McGowan P, Nelles N, Wimmer J, Williams D, Wen J, Li M, Ewton A, Curry C, Zu Y, Sheehan A, Chang CC (2012). "Differentiating between Burkitt lymphoma and CD10+ diffuse large B-cell lymphoma: the role of commonly used flow cytometry cell markers and the application of a multiparameter scoring system". Am. J. Clin. Pathol. 137 (4): 665–70. doi:10.1309/AJCP3FEPX5BEEKGX. PMC 4975158. PMID 22431545.
- Berglund M, Thunberg U, Amini RM, Book M, Roos G, Erlanson M, Linderoth J, Dictor M, Jerkeman M, Cavallin-Ståhl E, Sundström C, Rehn-Eriksson S, Backlin C, Hagberg H, Rosenquist R, Enblad G (2005). "Evaluation of immunophenotype in diffuse large B-cell lymphoma and its impact on prognosis". Mod. Pathol. 18 (8): 1113–20. doi:10.1038/modpathol.3800396. PMID 15920553. S2CID 7563005.
- Höller S, Horn H, Lohr A, Mäder U, Katzenberger T, Kalla J, Bernd HW, Went P, Ott MM, Müller-Hermelink HK, Rosenwald A, Ott G (2009). "A cytomorphological and immunohistochemical profile of aggressive B-cell lymphoma: high clinical impact of a cumulative immunohistochemical outcome predictor score". J Hematopathol. 2 (4): 187–94. doi:10.1007/s12308-009-0044-x. PMC 2798934. PMID 20309427.
- Xu Y, McKenna RW, Molberg KH, Kroft SH (2001). "Clinicopathologic analysis of CD10+ and CD10- diffuse large B-cell lymphoma. Identification of a high-risk subset with coexpression of CD10 and bcl-2". Am. J. Clin. Pathol. 116 (2): 183–90. doi:10.1309/J7RN-UXAY-55GX-BUNK. PMID 11488064.
- Fabiani B, Delmer A, Lepage E, Guettier C, Petrella T, Brière J, Penny AM, Copin MC, Diebold J, Reyes F, Gaulard P, Molina TJ (2004). "CD10 expression in diffuse large B-cell lymphomas does not influence survival". Virchows Arch. 445 (6): 545–51. doi:10.1007/s00428-004-1129-7. PMID 15517363. S2CID 23189608.
- Baseggio L, Traverse-Glehen A, Berger F, Ffrench M, Jallades L, Morel D, Goedert G, Magaud JP, Salles G, Felman P (2011). "CD10 and ICOS expression by multiparametric flow cytometry in angioimmunoblastic T-cell lymphoma". Mod. Pathol. 24 (7): 993–1003. doi:10.1038/modpathol.2011.53. PMID 21499231. S2CID 22353148.
- Yuan CM, Vergilio JA, Zhao XF, Smith TK, Harris NL, Bagg A (2005). "CD10 and BCL6 expression in the diagnosis of angioimmunoblastic T-cell lymphoma: utility of detecting CD10+ T cells by flow cytometry". Hum. Pathol. 36 (7): 784–91. doi:10.1016/j.humpath.2005.05.008. PMID 16084948.
- Attygalle AD, Diss TC, Munson P, Isaacson PG, Du MQ, Dogan A (2004). "CD10 expression in extranodal dissemination of angioimmunoblastic T-cell lymphoma". Am. J. Surg. Pathol. 28 (1): 54–61. doi:10.1097/00000478-200401000-00005. PMID 14707864. S2CID 25639416.
- Cook JR, Craig FE, Swerdlow SH (2003). "Benign CD10-positive T cells in reactive lymphoid proliferations and B-cell lymphomas". Mod. Pathol. 16 (9): 879–85. doi:10.1097/01.MP.0000084630.64243.D1. PMID 13679451. S2CID 21020212.
- Yasir S, Herrera L, Gomez-Fernandez C, Reis IM, Umar S, Leveillee R, Kava B, Jorda M (2012). "CD10+ and CK7/RON- immunophenotype distinguishes renal cell carcinoma, conventional type with eosinophilic morphology from its mimickers". Appl. Immunohistochem. Mol. Morphol. 20 (5): 454–61. doi:10.1097/PAI.0b013e31823fecd3. PMID 22417859. S2CID 1348915.
- Notohara K, Hamazaki S, Tsukayama C, Nakamoto S, Kawabata K, Mizobuchi K, Sakamoto K, Okada S (2000). "Solid-pseudopapillary tumor of the pancreas: immunohistochemical localization of neuroendocrine markers and CD10". Am. J. Surg. Pathol. 24 (10): 1361–71. doi:10.1097/00000478-200010000-00005. PMID 11023097. S2CID 3211829.
- Córdoba A, Guerrero D, Larrinaga B, Iglesias ME, Arrechea MA, Yanguas JI (2009). "Bcl-2 and CD10 expression in the differential diagnosis of trichoblastoma, basal cell carcinoma, and basal cell carcinoma with follicular differentiation". Int. J. Dermatol. 48 (7): 713–7. doi:10.1111/j.1365-4632.2009.04076.x. PMID 19570076. S2CID 205395744.
- Sari Aslani F, Akbarzadeh-Jahromi M, Jowkar F (2013). "Value of CD10 Expression in Differentiating Cutaneous Basal from Squamous Cell Carcinomas and Basal Cell Carcinoma from Trichoepithelioma". Iran J Med Sci. 38 (2): 100–6. PMC 3700055. PMID 23825889.
- Murali R, Delprado W (2005). "CD10 immunohistochemical staining in urothelial neoplasms". Am. J. Clin. Pathol. 124 (3): 371–9. doi:10.1309/04BH-F6A8-0BQM-H7HT. PMID 16191505.
- Bahadir B, Behzatoglu K, Bektas S, Bozkurt ER, Ozdamar SO (2009). "CD10 expression in urothelial carcinoma of the bladder". Diagn Pathol. 4 (1): 38. doi:10.1186/1746-1596-4-38. PMC 2780995. PMID 19917108.
- Mikami Y, Hata S, Kiyokawa T, Manabe T (2002). "Expression of CD10 in malignant müllerian mixed tumors and adenosarcomas: an immunohistochemical study". Mod. Pathol. 15 (9): 923–30. doi:10.1097/01.MP.0000026058.33869.DB. PMID 12218209. S2CID 30632093.
- McCluggage WG, Sumathi VP, Maxwell P (2001). "CD10 is a sensitive and diagnostically useful immunohistochemical marker of normal endometrial stroma and of endometrial stromal neoplasms". Histopathology. 39 (3): 273–8. doi:10.1046/j.1365-2559.2001.01215.x. PMID 11532038. S2CID 42287024.
- Kabbinavar FF, Hambleton J, Mass RD, Hurwitz HI, Bergsland E, Sarkar S (2005). "Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer". J. Clin. Oncol. 23 (16): 3706–12. doi:10.1200/JCO.2005.00.232. PMID 15867200.
- Weinreb I, Cunningham KS, Perez-Ordoñez B, Hwang DM (2009). "CD10 is expressed in most epithelioid hemangioendotheliomas: a potential diagnostic pitfall". Arch. Pathol. Lab. Med. 133 (12): 1965–8. doi:10.1043/1543-2165-133.12.1965 (inactive 2021-01-14). PMID 19961253.CS1 maint: DOI inactive as of January 2021 (link)
- Jung SM, Kuo TT (2005). "Immunoreactivity of CD10 and inhibin alpha in differentiating hemangioblastoma of central nervous system from metastatic clear cell renal cell carcinoma". Mod. Pathol. 18 (6): 788–94. doi:10.1038/modpathol.3800351. PMID 15578072. S2CID 16510470.
- Yin WH, Li J, Chan JK (2012). "Sporadic haemangioblastoma of the kidney with rhabdoid features and focal CD10 expression: report of a case and literature review". Diagn Pathol. 7 (1): 39. doi:10.1186/1746-1596-7-39. PMC 3364142. PMID 22497861.
External links
- The MEROPS online database for peptidases and their inhibitors: M13.001
- Neprilysin at the US National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: P08473 (Neprilysin) at the PDBe-KB.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.