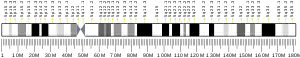ADAMTS2
A disintegrin and metalloproteinase with thrombospondin motifs 2 (ADAM-TS2) also known as procollagen I N-proteinase (PC I-NP) is an enzyme[5] that in humans is encoded by the ADAMTS2 gene.[6][7]
Gene
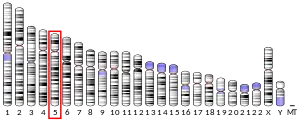
The ADAMTS2 gene is located on the long (q) arm of chromosome 5 at the end (terminus) of the arm, from base pair 178,473,473 to base pair 178,704,934.
Function
ADAMTS2 is responsible for processing several types of procollagen proteins. Procollagens are the precursors of collagens, the proteins that add strength and support to many body tissues. Specifically, this enzyme clips a short chain of amino acids off one end of the procollagen. This clipping step is necessary for collagen molecules to function normally and assemble into fibrils outside cells.
Clinical significance
Ehlers-Danlos syndrome, dermatosparaxis type is caused by mutations in the ADAMTS2 gene.[7] Several mutations in the ADAMTS2 gene have been identified in people with this syndrome. These mutations greatly reduce the production of the enzyme made by the ADAMTS2 gene. Procollagen cannot be processed correctly without this enzyme. As a result, collagen fibrils are not assembled properly; they appear ribbon-like and disorganized under the microscope. Cross-links, or chemical interactions, between collagen fibrils are also affected. These defects weaken connective tissue (the tissue that binds and supports the body's muscles, ligaments, organs, and skin), which causes the signs and symptoms of the disorder.
References
- ENSG00000283802 GRCh38: Ensembl release 89: ENSG00000087116, ENSG00000283802 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000036545 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Tang BL, Hong W (February 1999). "ADAMTS: a novel family of proteases with an ADAM protease domain and thrombospondin 1 repeats". FEBS Lett. 445 (2–3): 223–5. doi:10.1016/S0014-5793(99)00119-2. PMID 10094461. S2CID 37955930.
- "Entrez Gene: ADAM metallopeptidase with thrombospondin type 1 motif".
- Colige A, Nuytinck L, Hausser I, van Essen AJ, Thiry M, Herens C, Adès LC, Malfait F, Paepe AD, Franck P, Wolff G, Oosterwijk JC, Smitt JH, Lapière CM, Nusgens BV (October 2004). "Novel types of mutation responsible for the dermatosparactic type of Ehlers-Danlos syndrome (Type VIIC) and common polymorphisms in the ADAMTS2 gene". J. Invest. Dermatol. 123 (4): 656–63. doi:10.1111/j.0022-202X.2004.23406.x. PMID 15373769.
Further reading
- Wang WM, Lee S, Steiglitz BM, Scott IC, Lebares CC, Allen ML, Brenner MC, Takahara K, Greenspan DS (May 2003). "Transforming growth factor-beta induces secretion of activated ADAMTS-2. A procollagen III N-proteinase". J. Biol. Chem. 278 (21): 19549–57. doi:10.1074/jbc.M300767200. PMID 12646579.
- Reardon W, Winter RM, Smith LT, et al. (1995). "The natural history of human dermatosparaxis (Ehlers-Danlos syndrome type VIIC)". Clin. Dysmorphol. 4 (1): 1–11. doi:10.1097/00019605-199501000-00001. PMID 7735500. S2CID 2412884.
- Colige A, Vandenberghe I, Thiry M, et al. (2002). "Cloning and characterization of ADAMTS-14, a novel ADAMTS displaying high homology with ADAMTS-2 and ADAMTS-3". J. Biol. Chem. 277 (8): 5756–66. doi:10.1074/jbc.M105601200. PMID 11741898.
- Kevorkian L, Young DA, Darrah C, et al. (2004). "Expression profiling of metalloproteinases and their inhibitors in cartilage". Arthritis Rheum. 50 (1): 131–41. doi:10.1002/art.11433. PMID 14730609.
- Hurskainen TL, Hirohata S, Seldin MF, Apte SS (1999). "ADAM-TS5, ADAM-TS6, and ADAM-TS7, novel members of a new family of zinc metalloproteases. General features and genomic distribution of the ADAM-TS family". J. Biol. Chem. 274 (36): 25555–63. doi:10.1074/jbc.274.36.25555. PMID 10464288.
- Kimura K, Wakamatsu A, Suzuki Y, et al. (2006). "Diversification of transcriptional modulation: Large-scale identification and characterization of putative alternative promoters of human genes". Genome Res. 16 (1): 55–65. doi:10.1101/gr.4039406. PMC 1356129. PMID 16344560.
- Dubail J, Kesteloot F, Deroanne C, et al. (2010). "ADAMTS-2 functions as anti-angiogenic and anti-tumoral molecule independently of its catalytic activity" (PDF). Cellular and Molecular Life Sciences. 67 (24): 4213–32. doi:10.1007/s00018-010-0431-6. PMID 20574651. S2CID 20047628.
- Colige A, Sieron AL, Li SW, et al. (1999). "Human Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene". Am. J. Hum. Genet. 65 (2): 308–17. doi:10.1086/302504. PMC 1377929. PMID 10417273.
- Hartley JL, Temple GF, Brasch MA (2000). "DNA Cloning Using In Vitro Site-Specific Recombination". Genome Res. 10 (11): 1788–95. doi:10.1101/gr.143000. PMC 310948. PMID 11076863.
- Tang BL (2001). "ADAMTS: a novel family of extracellular matrix proteases". Int. J. Biochem. Cell Biol. 33 (1): 33–44. doi:10.1016/S1357-2725(00)00061-3. PMID 11167130.
- Lasky-Su J, Anney RJ, Neale BM, et al. (2008). "Genome-wide association scan of the time to onset of Attention Deficit Hyperactivity Disorder". Am. J. Med. Genet. B Neuropsychiatr. Genet. 147B (8): 1355–8. doi:10.1002/ajmg.b.30869. PMC 2605611. PMID 18937294.
- Colige A, Ruggiero F, Vandenberghe I, et al. (2005). "Domains and maturation processes that regulate the activity of ADAMTS-2, a metalloproteinase cleaving the aminopropeptide of fibrillar procollagens types I-III and V". J. Biol. Chem. 280 (41): 34397–408. doi:10.1074/jbc.M506458200. PMID 16046392.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2002). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Brandenberger R, Wei H, Zhang S, et al. (2004). "Transcriptome characterization elucidates signaling networks that control human ES cell growth and differentiation". Nat. Biotechnol. 22 (6): 707–16. doi:10.1038/nbt971. PMID 15146197. S2CID 27764390.
- Tomii Y, Kamochi J, Yamazaki H, et al. (2002). "Human thrombospondin 2 inhibits proliferation of microvascular endothelial cells". Int. J. Oncol. 20 (2): 339–42. doi:10.3892/ijo.20.2.339. PMID 11788898.