Asunaprevir
Asunaprevir (formerly BMS-650032, brand name in Japan and Russia[1] Sunvepra) is an experimental drug candidate for the treatment of hepatitis C. It is undergoing development by Bristol-Myers Squibb and is currently in Phase III clinical trials.[2]
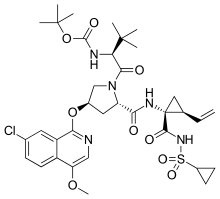 | |
| Names | |
|---|---|
| IUPAC name
3-Methyl-N-{[(2-methyl-2-propanyl)oxy]carbonyl}-L-valyl-(4R)-4-[(7-chloro-4-methoxy-1-isoquinolinyl)oxy]-N-{(1R,2S)-1-[(cyclopropylsulfonyl)carbamoyl]-2-vinylcyclopropyl}-L-prolinamide | |
| Other names
BMS-650032 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.206.482 |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C35H46ClN5O9S | |
| Molar mass | 748.29 g·mol−1 |
| Pharmacology | |
| J05AP06 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Asunaprevir is an inhibitor of the hepatitis C virus enzyme serine protease NS3.[3]
Asunaprevir is being tested in combination with pegylated interferon and ribavirin, as well as in interferon-free regimens with other direct-acting antiviral agents including daclatasvir.[4][5][6]
See also
References
- "Sunvepra™ (asunaprevir) soft gelatin capsules 100 mg. Registration certificate". State Register of Medicines (in Russian). Retrieved 26 August 2015.
- "A Phase 3 Study in Combination With BMS-790052 and BMS-650032 in Japanese Hepatitis C Virus (HCV) Patients". ClinicalTrials.gov.
- C. Reviriego (2012). "Asunaprevir. HCV serine protein NS3 inhibitor, Treatment of hepatitis C virus". Drugs of the Future. 37 (4): 247–254. doi:10.1358/dof.2012.037.04.1789350.
- Lok, A; et al. (2012). "Preliminary Study of Two Antiviral Agents for Hepatitis C Genotype 1". New England Journal of Medicine. 366 (3): 216–224. doi:10.1056/NEJMoa1104430. PMID 22256805.
- "Bristol-Myers' Daclatasvir, Asunaprevir Cured 77%: Study". Bloomberg. Apr 19, 2012.
- AASLD: Daclatasvir plus Asunaprevir Rapidly Suppresses HCV in Prior Null Responders Archived 2015-02-08 at the Wayback Machine. Highleyman, L. HIVandHepatitis.com. 8 November 2011.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.