Setrobuvir
Setrobuvir (also known as ANA-598) was an experimental drug candidate for the treatment of hepatitis C that was discovered at Anadys Pharmaceuticals, which was acquired by Roche in 2011; Roche terminated development in July 2015.[2][3] It was in Phase IIb clinical trials, used in combination with interferon and ribavirin, targeting hepatitis C patients with genotype 1.[3]
 | |
| Names | |
|---|---|
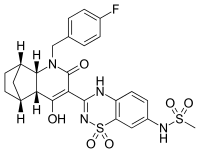
| IUPAC name
N-(3-{(4aR,5S,8R,8aS)-1-[(4-fluorophenyl)methyl]-4-hydroxy-2-oxo-1,2,4a,5,6,7,8,8a-octahydro-5,8-methanoquinolin-3-yl}-1,1-dioxo-1,4-dihydro-1λ6,2,4-benzothiadiazin-7-yl)methanesulfonamide[1] | |
| Other names
ANA-598; ANA598 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C25H25FN4O6S2 | |
| Molar mass | 560.62 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Setrobuvir works by inhibiting the hepatitis C enzyme NS5B, an RNA polymerase.[4]
References
- "International Nonproprietary Names for Pharmaceutical Substances (INN). RECOMMENDED International Nonproprietary Names: List 68" (PDF). World Health Organization. p. 322.
- "Setrobuvir". AdisInsight. Retrieved 28 August 2017.
- "HCV Followup: Anadys Acquired for Active Antiviral". Chemical & Engineering News. October 24, 2011.
- Ruebsam, F; Murphy, DE; Tran, CV; Li, LS; Zhao, J; Dragovich, PS; McGuire, HM; Xiang, AX; et al. (2009). "Discovery of tricyclic 5,6-dihydro-1H-pyridin-2-ones as novel, potent, and orally bioavailable inhibitors of HCV NS5B polymerase". Bioorganic & Medicinal Chemistry Letters. 19 (22): 6404–12. doi:10.1016/j.bmcl.2009.09.045. PMID 19818610.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.