Pimodivir
Pimodivir (VX-787, JNJ-63623872) is an antiviral drug which was developed as a treatment for influenza. It acts as an inhibitor of influenza virus polymerase basic protein 2, and has shown promising results in Phase II clinical trials.[1][2][3]
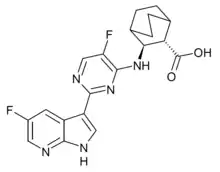 | |
| Clinical data | |
|---|---|
| Trade names | Pimodivir |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H19F2N5O2 |
| Molar mass | 399.4 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- Trevejo JM, Asmal M, Vingerhoets J, Polo R, Robertson S, Jiang Y, et al. (2018). "Pimodivir treatment in adult volunteers experimentally inoculated with live influenza virus: a Phase IIa, randomized, double-blind, placebo-controlled study". Antiviral Therapy. 23 (4): 335–344. doi:10.3851/IMP3212. PMID 29244026.
- Finberg RW, Lanno R, Anderson D, Fleischhackl R, van Duijnhoven W, Kauffman RS, et al. (March 2019). "Phase 2b Study of Pimodivir (JNJ-63623872) as Monotherapy or in Combination With Oseltamivir for Treatment of Acute Uncomplicated Seasonal Influenza A: TOPAZ Trial". The Journal of Infectious Diseases. 219 (7): 1026–1034. doi:10.1093/infdis/jiy547. PMID 30428049.
- Beigel JH, Nam HH, Adams PL, Krafft A, Ince WL, El-Kamary SS, Sims AC (July 2019). "Advances in respiratory virus therapeutics - A meeting report from the 6th isirv Antiviral Group conference". Antiviral Research. 167: 45–67. doi:10.1016/j.antiviral.2019.04.006. PMC 7132446. PMID 30974127.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
