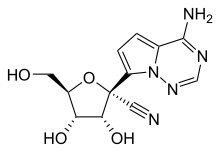
GS-441524
GS-441524 is a nucleoside analogue antiviral drug which was developed by Gilead Sciences. It is the main plasma metabolite of the antiviral prodrug remdesivir, and has a half-life of around 24 hours in human patients. Remdesivir and GS-441524 were both tested against feline infectious peritonitis (FIP) in cell culture and found to be equivalent. Remdesivir was never tested in cats but GS-441524 has been found to be effective treatment for FIP, a lethal coronavirus disease which affects domestic cats and is widely used despite no official FDA approval due to Gilead's refusal to license this drug for veterinary use.[1][2][3][4]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C12H13N5O4 |
| Molar mass | 291.26 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Use and research
Since FIP is usually fatal and there are no approved treatments available, GS-441524 has reportedly been sold on the black market and used by pet owners to treat affected cats, although Gilead Sciences has refused to license the drug for veterinary use. Its efficacy for this purpose has been conclusively demonstrated in multiple trials, including field trials.[1][2][5]
GS-441524 is either similar to or more potent than remdesivir against SARS-CoV-2 in cell culture,[6] with some researchers arguing that GS-441524 would be better than remdesivir for the treatment of COVID-19.[1][7][8][9] Specific advantages cited include ease of synthesis, lower kidney and hepatotoxicity, as well as potential for oral delivery (which is precluded of remdesivir because of poor hepatic stability and first pass metabolism).[10] The public health advocacy group, Public Citizen, in an open letter urged the DHHS and Gilead to investigate GS-441524 for the treatment of COVID-19,[11] suggesting that GILEAD was not doing so for financial motives related to the longer intellectual property lifespan of Remdesivir. Direct efficacy against SARS-CoV-2 was demonstrated in a mouse model of COVID-19.[12] A deuterium modified version of GS-441524 has been produced and has shown pre-clinical efficacy in both cell culture and mouse models by a team including members of Wuhan Institute of Virology.[13]
GS-441524 is sold as a research chemical in very high purity (>99% by NMR and HPLC) by a number of suppliers including MedKoo, Selleckchem and MedChemExpress. Such sales for research purposes do not constitute patent infringements which was affirmed by a supreme court decision. However, despite the high purity, under FDA regulations, such chemicals are not allowed for clinical trials since their manufacture is not performed under FDA cGMP certified conditions. Such chemicals, like any chemicals, may however be administered to patients at an individual clinician's discretion in practice (not as part of a formal trial); however harms resulting from such use are not as well legally protected from malpractice claims as is use of approved ethical pharmaceutical drugs.
Pharmacology
Pharmacodynamics
GS-441524 nucleoside is phosphorylated by nucleoside kinases (probably adenosine kinase (ADK), which is the enzyme that phosphorylates the structurally similar ribavirin), and then phosphorylated again by nucleoside-diphosphate kinase (NDK) to the active nucleotide triphosphate form.
Pharmacokinetics
GS-441524 is the nucleoside of the prodrug remdesivir. It is remdesivir's predominant metabolite circulating in the serum due to rapid hydrolysis (half life less than 1 hour) followed by dephosphorylation.[14][15][16] Some researchers have suggested its utility as a treatment for COVID-19 and pointed out advantages over remdesivir, including lack of on-target liver toxicity, longer half-life and exposure (AUC) and much cheaper and simpler synthesis.[7][8][17]
In response to the letter from Public citizen, NIH's drug discovey arm, NCATS has started systematic IND enabling experiments including PK in multiple pre-clinical species, and also (in October) in humans (results not yet published). Oral bioavailability was found to be excellent in dogs, good in mice, but modest in cynomolgus non-human primates. Prediction of human oral bioavailability from pre-clinical data is more art than science, and relies on modeling data from multiple species. Taking as reference point the clinical and pre-clinical data of other nucleoside analogues, human oral bioavailability of GS-441524 is expected to fall somewhere in between that seen in dog as a high point and that seen in non-human primates. Since GS-441524 has a bit less than half the molecular weight of remdesivir, it will deliver as much active metabolite to the blood as the same dose of remdesivir (for example, 100 mg), even if human oral bioavailability is 50%, comparable to (for example) ribivirin. [18]
The elimination half-life of GS-441524 is around 2 hours in cynomolgus, much shorter than the 24 hours reported in humans. The longer half life suggests once-a-day dosing if the drug is approved for human oral use.
References
- Westgate, James (7 May 2020). "Vet science 'being ignored' in quest for COVID-19 drug". vet times. Retrieved 6 July 2020.
- Zhang, Sarah (8 May 2020). "A Much-Hyped COVID-19 Drug Is Almost Identical to a Black-Market Cat Cure". The Atlantic. Retrieved 6 July 2020.
- Murphy BG, Perron M, Murakami E, Bauer K, Park Y, Eckstrand C, Liepnieks M, Pedersen NC (June 2018). "The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies". Veterinary Microbiology. 219: 226–233. doi:10.1016/j.vetmic.2018.04.026. PMC 7117434. PMID 29778200.
- Pedersen NC, Perron M, Bannasch M, Montgomery E, Murakami E, Liepnieks M, Liu H (April 2019). "Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis". Journal of Feline Medicine and Surgery. 21 (4): 271–281. doi:10.1177/1098612X19825701. PMC 6435921. PMID 30755068.
- Burns, Katie (15 January 2020). "FIP drugs continue to show promise, while being sold on black market". JAVMAnews. Retrieved 2 May 2020.
- Pruijssers AJ, George AS, Schäfer A, Leist SR, Gralinksi LE, Dinnon KH, Yount BL, Agostini ML, Stevens LJ, Chappell JD, Lu X, Hughes TM, Gully K, Martinez DR, Brown AJ, Graham RL, Perry JK, Du-Point V, Pitts J, Ma B, Babusis D, Murakami E, Feng JY, Bilello JP, Porter DP, Cihlar T, Baric RS, Denison MR, Sheahan TP (21 July 2020). "Remdesivir potently inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice". Cell Reports. 32 (3): 107940. doi:10.1016/j.celrep.2020.107940. PMC 7340027. PMID 32668216. Retrieved 5 July 2020.
- Yan VC, Muller FL (23 June 2020). "Advantages of the parent nucleoside GS-441524 over remdesivir for Covid-19 treatment". ACS Medicinal Chemistry Letters. 11 (7): 1361–1366. doi:10.1021/acsmedchemlett.0c00316. PMC 7315846. PMID 32665809. S2CID 220056568.
- Yan, V.C.; Muller, F.L. (14 May 2020). "Gilead should ditch remdesivir and focus on its simpler and safer ancestor". Statnews. Retrieved 5 July 2020.
- Siebenand, Sven (15 April 2020). "Remdesivir-Metabolit noch schärfere Waffe gegen Covid-19?". Pharmazeutische Zeitung. Retrieved 6 July 2020.
- "Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Veklrty® (remdesivir)". fda.gov. Food and Drug Administration. July 2020. Retrieved 15 August 2020.
- "Letter to Gilead and Senior Federal Health Officials Calling for Immediate Study of the Antiviral Drug GS-441524 as a Potential Treatment for COVID-19" (Press release). Public Citizen. Public Citizen. 4 August 2020. Retrieved 15 August 2020.
- https://www.biorxiv.org/content/10.1101/2020.10.26.353300v1
- https://www.biorxiv.org/content/10.1101/2020.11.01.363812v1
- Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, Siegel D, Perron M, Bannister R, Hui HC, Larson N, Strickley R, Wells J, Stuthman KS, Van Tongeren SA, Garza NL, Donnelly G, Shurtleff AC, Retterer CJ, Gharaibeh D, Zamani R, Kenny T, Eaton BP, Grimes E, Welch LS, Gomba L, Wilhelmsen CL, Nichols DK, Nuss JE, Nagle ER, Kugelman JR, Palacios G, Doerffler E, Neville S, Carra E, Clarke MO, Zhang L, Lew W, Ross B, Wang Q, Chun K, Wolfe L, Babusis D, Park Y, Stray KM, Trancheva I, Feng JY, Barauskas O, Xu Y, Wong P, Braun MR, Flint M, McMullan LK, Chen SS, Fearns R, Swaminathan S, Mayers DL, Spiropoulou CF, Lee WA, Nichol ST, Cihlar T, Bavari S (17 March 2016). "Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys". Nature. 531 (7594): 381–385. doi:10.1038/nature17180. PMC 5551389. PMID 26934220.
- Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I, Bannister R, Park Y, Babusis D, Clarke MO, Mackman RL, Spahn JE, Palmiotti CA, Siegel D, Ray AS, Cihlar T, Jordan R, Denison MR, Baric RS (28 June 2017). "Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses". Science Translational Medicine. 9 (396): eaal3653. doi:10.1126/scitranslmed.aal3653. PMC 5567817. PMID 28659436.
- Williamson BN, Feldmann F, Schwarz B, Meade-white K, Porter DP, Schulz J, van Doremalen N, Leighton I, Yinda CK, Pérez-Pérez L, Okumura A, Lovaglio J, Hanley PW, Saturday G, Bosio CM, Anzick S, Barbian K, Cihlar T, Martens C, Scott DP, Munster VJ, de Wit E (22 April 2020). "Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2". bioRxiv: 2020.04.15.043166. doi:10.1101/2020.04.15.043166. PMC 7239049. PMID 32511319.
- https://www.fox5ny.com/news/feline-coronavirus-treatment-could-stop-spread-of-covid-19-in-humans-doctor-says
- WP:CALC