Beer chemistry
The chemical compounds in beer give it a distinctive taste, smell and appearance. The majority of compounds in beer come from the metabolic activities of plants and yeast and so are covered by the fields of biochemistry and organic chemistry.[1] The main exception is that beer contains over 90% water and the mineral ions in the water (hardness) can have a significant effect upon the taste.[2]

Ingredients
Four main ingredients are used to make beer in the process of brewing.
Carbohydrate
The carbohydrate source is an essential part of the beer because unicellular yeast organisms convert carbohydrates into energy to live. Yeast metabolize the carbohydrate source to form a number of compounds including ethanol. The process of brewing beer starts with malting and mashing, which breaks down the long carbohydrates in the barley grain into more simple sugars. This is important because yeast can only metabolize very short chains of sugars.[3] Long-carbohydrates are polymers, large branching linkages of the same molecule over and over. In the case of barley, we mostly see polymers called amylopectin and amylose which are made of repeating linkages of glucose. On very large time-scales (thermodynamically) these polymers would break down on their own, and there would be no need for the malting process.[4] The process is normally sped up by heating up the barley grain.[3] This heating process activates enzymes called amylases. The shape of these enzymes, their active site, gives them the unique and powerful ability to speed these degradation reactions to over 100,000 times faster. The reaction that takes place at the active site is called a hydrolysis reaction, which is a cleavage of the linkages between the sugars. Repeated hydrolysis breaks the long amylopectin polymers into simpler sugars that can be digested by the yeast.[4]
Hops
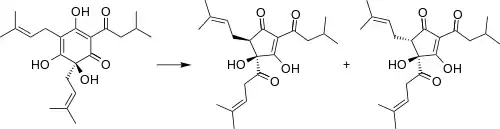
Hops are the flowers of the hops plant Humulus lupulus. These flowers contain over 440 essential oils, which contribute to the aroma and non-bitter flavors of beer.[4] However, the distinct bitterness especially characteristic of pale ales comes from a family of compounds called alpha-acids (also called humulones) and beta-acids (also called lupulones). Generally, brewers believe that α-acids give the beer a pleasant bitterness whereas β-acids are considered less pleasant.[4] α-acids isomerize during the boiling process in the reaction pictured. The six-member ring in the humulone isomerizes to a five-member ring, but it is not commonly discussed how this affects perceived bitterness.
Yeast
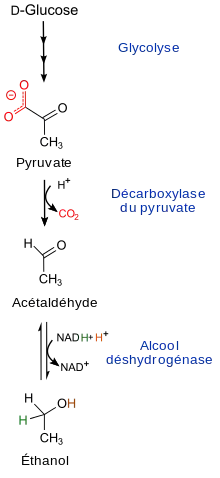
In beer, the metabolic waste of yeast is a significant factor. In aerobic conditions, the yeast will use the simple sugars from the malting process in glycolysis, and send the major organic product of glycolysis (pyruvate) into carbon dioxide and water via cellular respiration, many homebrewers use this aspect of yeast metabolism to carbonate their beers. However, under anaerobic conditions yeast cannot use the end products of glycolysis to generate energy in cellular respiration. Instead, they rely on a process called fermentation. Fermentation converts pyruvate into ethanol through the intermediate acetaldehyde.
Water
Water can often play a very important role in the way a beer tastes,[2][4] as it is the main ingredient. The ion varieties present in water can affect the metabolic pathways of yeast, and thus the metabolites one can taste. For example, calcium and iron are essential in small amounts for yeast to survive, because these metals are usually required cofactors for yeast enzymes.[4]
Carbonation
In aerobic conditions, yeast turns sugars into pyruvate then converts pyruvate into water and carbon dioxide. This process can carbonate beers. In commercial production, the yeast works in anaerobic conditions to convert pyruvate into ethanol, and does not carbonate beer. Beer is carbonated with pressurized CO2. When beer is poured, carbon dioxide that is dissolved in the beer forms bubbles. These bubbles grow and accelerate as they rise by feeding off of nearby smaller bubbles, a phenomenon known as Ostwald ripening. These larger bubbles lead to “coarser” foam on top of poured beer.
Nitro beer
Beers can be carbonated with CO2 or with other gases such as Nitrogen. These gases are not as soluble in water as carbon dioxide, so they form bubbles that do not grow through Ostwald ripening. This means that the beer has smaller bubbles and a more creamy and stable head.[6] This less soluble gas gives the beer a different and flatter texture. In beer terms, the mouthfeel is smooth, not bubbly like beers with normal carbonation. Nitro beer could taste less acidic than normal beer.[7]
Storage and degradation
A particular problem with beer is that, unlike wine, its quality tends to deteriorate as it ages. A cat urine smell and flavor called ribes, named for the genus of the black currant, tends to develop and peak.[8] A cardboard smell then dominates which is due to the release of 2-Nonenal.[9] In general, chemists believe that the "off-flavors" that come from old beers are due to reactive oxygen species. These may come in the form of oxygen free radicals, for example, which can change the chemical structures of compounds in beer that give them their taste.[9] Oxygen radicals can cause increased concentrations of aldehydes from the Strecker degradation reactions of amino acids in beer.[10]
Beer is unique when compared to other alcoholic drinks because it is unstable in the final package. There are many variables and chemical compounds that affect the flavor of beer during the production steps, but also during the storage of beer. Beer will develop an off-flavor during storage because of many factors, including sunlight and the amount of oxygen in the headspace of the bottle. Other than changes in taste, beer can also develop visual changes. Beer can become hazy during storage. This is called colloidal stability (haze formation) and is typically caused by the raw materials used during the brewing process. The primary reaction that causes beer haze is the polymerization of polyphenols and binding with specific proteins. This type of haze can be seen when beer is cooled below 0 degrees Celsius. When the beer is raised to room temperature, the haze dissolves. But if a beer is stored at room temperature for too long (about 6 months) a permanent haze will form.[11] A study done by Heuberger et al. concludes that storage temperature of beers affects the flavor stability. They found that the metabolite profile of room temperature and cold temperature stored beer differed significantly from fresh beer. They also have evidence to support significant beer oxidation after weeks of storage, which also has an effect on the flavor of beer.[12]
The off-flavour in beer, such as a cardboard or green apple taste, is often associated with the appearance of staling aldehydes. The Strecker aldehydes responsible for the flavor change are formed during storage of the beers. Philip Wietstock et al. performed experiments to test what causes the formation of Strecker aldehydes during storage. They found that only amino acid concentration (Leu, Ile, and Phe, specifically) and oxygen concentration caused Strecker aldehyde formation. They also tested carbohydrate and Fe2+ additions. A linear relationship was found between Strecker aldehydes formed and total packaged oxygen.1 This is important for brewers to know so that they can control the taste of their beer. Wietstock concludes that capping beers with oxygen barrier crown corks will diminish Strecker aldehyde formation.[10]
In another study done by Vanderhaegen et al., different aging conditions were tested on a bottled beer after 6 months. They found a decrease in volatile esters was responsible for a reduced fruity flavor. They also found an increase in many other compounds including carbonyl compounds, ethyl esters, Maillard compounds, dioxolanes, and furanic ethers.[13] The carbonyl compounds, as stated previously in the Wietstock experiments, will create Strecker aldehydes, which tend to cause a green apple flavor. Esters are known to cause fruity flavors such as pears, roses, and bananas. Maillard compounds will cause a toasty, malty flavor.
A study done by Charles Bamforth and Roy Parsons also confirms that beer staling flavors are caused by various carbonyl compounds. They used thiobarbituric acid (TBA) to estimate the staling substances after using an accelerated aging technique. They found that beer staling is reduced by scavengers of the hydroxyl radical, such as mannitol and ascorbic acid. They also tested the hypothesis that soybean extracts included in the fermenting wort enhance the shelf life of beer flavor.[14]
See also
References
Citations
- Barth 2013, p. 9,89.
- Barth 2013, p. 69-88.
- Barth 2013, p. 144.
- Janson 1996.
- Marnett, Alan, "The Chemistry of Skunky Beer", benchfly.com
- Craig Bettenhausen (2015), "Helium Beer, From Prank To Tank.", Chemical & Engineering News Archive, vol. 93 (43): 56, doi:10.1021/cen-09343-newscripts, ISSN 1520-605X
- Lisa Jarvis; Jessica Morrison (2015), "Nitro Cold Brew.", Chemical & Engineering News Archive, vol. 93 (33): 37, doi:10.1021/cen-09343-newscripts, ISSN 1520-605X
- Barth 2013, p. 231.
- Bart Vanderhaegen; Hedwig Neven; Hubert Verachtert; Guy Derdelinckx (2006), "The chemistry of beer aging – a critical review", Food Chemistry, vol. 95 (3): 357–381, doi:10.1016/j.foodchem.2005.01.006, ISSN 0308-8146
- Wietstock, Philip C.; Kunz, Thomas; Methner, Frank-Jürgen (2016), "Relevance of Oxygen for the Formation of Strecker Aldehydes during Beer Production and Storage", Journal of Agricultural and Food Chemistry, 64 (42): 8035–8044, doi:10.1021/acs.jafc.6b03502, ISSN 1520-5118
- Stewart, Graham (July 1, 2004). "The Chemistry of Beer Instability". Journal of Chemical Education. 7 (81): 963. doi:10.1021/ed081p963.
- Hueberger, Adam; Broeckling, Corey; Lewis, Matthew; Salazar, Lauren; Bouckaert, Peter; Prenni, Jessica (1 December 2012). "Metabolomic profiling of beer reveals effect of temperature on non-volatile small molecules during short-term storage". Food Chemistry. 135 (3): 284–1289. doi:10.1016/j.foodchem.2012.05.048.
- Vanderhaegen, Bart; Neven, Hedwig; Coghe, Stefan; Verachtert, Verstrepen; erdelinckx, Guy (2003). "Evolution of Chemical and Sensory Properties during Aging of Top-Fermented Beer". Journal of Agricultural and Food Chemistry. 51 (23): 6782–6790. doi:10.1021/jf034631z.
- Bamforth, Charles; Parsons, Roy (1985). "New Procedures to Improve the Flavor Stability of Beer". Journal of the ASBC. 43 (0197). doi:10.1094/ASBCJ-43-0197.
Sources
- Barth, Roger (2013), The Chemistry of Beer: The Science in the Suds, John Wiley & Sons, ISBN 978-1-11873-379-0
- Baxter, Denise; Hughes, Paul (2001), Beer: Quality, Safety and Nutritional Aspects, Royal Society of Chemistry, ISBN 978-0-85404-588-4
- Hopkins, R (2011), Biochemistry Applied to Beer Brewing – General Chemistry of the Raw Materials of Malting and Brewing, Tobey Press, ISBN 978-1-44654-168-5
- Hornsey, Ian (2003), A History of Beer and Brewing, Royal Society of Chemistry, ISBN 978-0-85404-630-0
- Janson, Lee (1996), Brew Chem 101, Storey, ISBN 978-0-88266-940-3
- Verhagen, Leen (2010), "Beer Flavor", Comprehensive Natural Products II: Chemistry and Biology, Newnes, Vol. 3: Development & Modification of Bioactivity, pp. 967–998, ISBN 978-0-08045-382-8
- Verzele, M; De Keukeleire, D (2013), Chemistry and Analysis of Hop and Beer Bitter Acids, Elsevier, ISBN 978-1-48329-086-7
External links
- Tapping into the Chemistry of Beer and Brewing—an online lecture by Charles Bamforth, Professor of Malting & Brewing Sciences at the University of California
- Chemistry of Beer—an online course in the subject at the University of Oklahoma