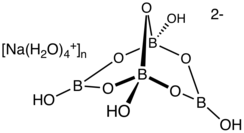
Borax
Borax, also known as sodium borate, sodium tetraborate, or disodium tetraborate, is a compound with formula Na
2H
4B
4O
9•nH
2O or, more precisely, [Na•(H
2O)+
m]
2 [B
4O
5(OH)2−
4].[9][10]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Sodium tetraborate decahydrate | |
| Other names
Borax octahydrate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| E number | E285 (preservatives) |
| KEGG | |
PubChem CID |
|
| UNII |
|
| |
| |
| Properties | |
| Na2B4O7·10H2O or Na2[B4O5(OH)4]·8H2O | |
| Molar mass | 201.22 (anhydrous) 381.38 (decahydrate) |
| Appearance | white solid |
| Density | 2.4 g/cm3 (anhydrous, solid)[1] 1.73 g/cm3 (decahydrate, solid)[1] |
| Melting point | 743 °C (1,369 °F; 1,016 K) (anhydrous)[1] 75 °C (decahydrate, decomposes)[1] |
| Boiling point | 1,575 °C (2,867 °F; 1,848 K) (anhydrous)[1] |
| 31.7 g/L (both)[1] | |
| −85.0·10−6 cm3/mol (anhydrous)[2] | |
Refractive index (nD) |
n1=1.447, n2=1.469, n3=1.472 (decahydrate)[3] |
| Structure[4] | |
| Monoclinic, mS92, No. 15 | |
| C2/c | |
| 2/m | |
a = 1.1885 nm, b = 1.0654 nm, c = 1.2206 nm α = 90°, β = 106.623°, γ = 90° | |
Lattice volume (V) |
1.4810 nm3 |
Formula units (Z) |
4 |
| Pharmacology | |
| S01AX07 (WHO) | |
| Hazards | |
| GHS pictograms |  |
| H360 | |
| P201, P308+313 | |
| NFPA 704 (fire diamond) | |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
none[6] |
REL (Recommended) |
TWA 1 mg/m3 (anhydrous and pentahydrate)[6][7] TWA 5 mg/m3 (decahydrate)[8] |
IDLH (Immediate danger) |
N.D.[6] |
| Related compounds | |
Other anions |
Sodium aluminate |
Other cations |
Lithium tetraborate |
Related compounds |
Boric acid, sodium perborate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The formula is often improperly written as Na
2B
4O
7•(n+2)H
2O, reflecting an older incorrect understanding of the anion's molecular structure. The name may refer to any of a number of closely related boron-containing mineral or chemical compounds that differ in their water of crystallization content. The most commonly encountered one is the octahydrate Na
2H
4B
4O
9•8H
2O or [Na(H
2O)+
4]
2 [B
4O
5(OH)2−
4] (or Na
2B
4O
7•10H
2O, the "decahydrate", in the older notation).[9] It is a colorless crystalline solid that dissolves in water.
Borax is a component of many detergents, cosmetics, and enamel glazes. It is used to make buffer solutions in biochemistry, as a fire retardant, as an anti-fungal compound, in the manufacture of fiberglass, as a flux in metallurgy, neutron-capture shields for radioactive sources, a texturing agent in cooking, as a cross-linking agent in slime, as an alkali in photographic developers, as a precursor for other boron compounds, and is useful as an insecticide (similarly to boric acid).
In artisanal gold mining, borax is sometimes used as part of a process (as a flux) meant to eliminate the need for toxic mercury in the gold extraction process, although it cannot directly replace mercury. Borax was reportedly used by gold miners in parts of the Philippines in the 1900s.[11]
Borax was first discovered in dry lake beds in Tibet and was imported via the Silk Road to the Arabian Peninsula in the 8th century AD.[12] Borax first came into common use in the late 19th century when Francis Marion Smith's Pacific Coast Borax Company began to market and popularize a large variety of applications under the 20 Mule Team Borax trademark, named for the method by which borax was originally hauled out of the California and Nevada deserts.[13][14]
Chemistry
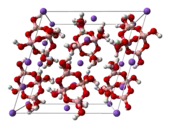
Structure
The term borax is often used for a number of closely related minerals or chemical compounds that differ in their crystal water content:
- anhydrous sodium tetraborate, Na2B4O7
- sodium tetraborate pentahydrate, Na2B4O7·5H2O
- sodium tetraborate decahydrate, Na2B4O7·10H2O or equivalently the octahydrate, Na2B4O5(OH)4·8H2O
From the chemical perspective, borax contains the [B4O5(OH)4]2− ion. In this structure, there are two four-coordinate boron centers and two three-coordinate boron centers.
Reactions
Borax is also easily converted to boric acid and other borates, which have many applications. Its reaction with hydrochloric acid to form boric acid is:
- Na2B4O7·10H2O + 2 HCl → 4 B(OH)3 + 2 NaCl + 5H2O
The "decahydrate" is sufficiently stable to find use as a primary standard for acid base titrimetry.[15]
When borax is added to a flame, it produces a yellow green color.[16] Borax is not used for this purpose in fireworks due to the overwhelming yellow color of sodium. Boric acid is used to color methanol flames a transparent green.
Borax is very soluble in ethylene glycol, moderately soluble in diethylene glycol and methanol, slightly soluble in acetone.[17] It is poorly soluble in cold water, but its solubility increases significantly with temperature.[17][18]
Etymology
The English word borax is Latinized: the Middle English form was boras, from Old French boras, bourras. That may have been from medieval Latin baurach (another English spelling), borac(-/um/em), borax, along with Spanish borrax (> borraj) and Italian borrace, in the 9th century. Another name for borax is tincal, from Sanskrit.[12]
The word tincal /ˈtɪŋkəl/ "tinkle", or tincar /ˈtɪŋkər/ "tinker", refers to crude borax, before it is purified, as mined from lake deposits in Tibet, Persia, and other parts of Asia. The word was adopted in the 17th century from Malay tingkal and from Urdu/Persian/Arabic تنکار tinkār/tankār; thus the two forms in English. These all appear to be related to the Sanskrit टांकण ṭānkaṇa.[19][20]
Natural sources

Borax occurs naturally in evaporite deposits produced by the repeated evaporation of seasonal lakes. Fibrous nodules of ulexite and proberite (sodium and calcium borate minerals) known as "cottonball" were found in salt deposits on the valley floor of California's Death Valley in the 1880s and taken to market on 20-mule team wagons. By the 1890s railroads were transporting purer forms of mined borax such as colemanite, kernite, priceite, and ulexite.[21]
The most commercially important deposits are found in: Turkey; Boron, California; and Searles Lake, California. Borax has been found at many other locations in the Southwestern United States, the Atacama desert in Chile, and in Bolivia, Tibet, and Romania.
Naturally occurring borax is refined by a process of recrystallization.[22]
Borax can be produced synthetically from other boron compounds.
Uses

Household products
Borax is used in various household laundry and cleaning products,[23] including the "20 Mule Team Borax" laundry booster, "Boraxo" powdered hand soap, and some tooth bleaching formulas.[24]
pH buffer
Borate ions (commonly supplied as boric acid) are used in biochemical and chemical laboratories to make buffers, e.g. for polyacrylamide gel electrophoresis of DNA and RNA, such as TBE buffer (borate buffered tris-hydroxymethylaminomethonium)[25][26] or the newer SB buffer or BBS buffer (borate buffered saline) in coating procedures. Borate buffers (usually at pH 8) are also used as preferential equilibration solution in dimethyl pimelimidate (DMP) based crosslinking reactions.
Co-complexing agent
Borax as a source of borate has been used to take advantage of the co-complexing ability of borate with other agents in water to form complex ions with various substances. Borate and a suitable polymer bed are used to chromatograph non-glycosylated hemoglobin differentially from glycosylated hemoglobin (chiefly HbA1c), which is an indicator of long-term hyperglycemia in diabetes mellitus.
Water-softening agent
Borax alone does not have a high affinity for the hardness cations, although it has been used for water-softening. Its chemical equation for water-softening is given below:
- Ca2+ (aq) + Na2B4O7 (aq) → Ca B4O7 (s)↓ + 2 Na+ (aq)
- Mg2+ (aq) + Na2B4O7 (aq) → Mg B4O7 (s)↓ + 2 Na+ (aq)
The sodium ions introduced do not make water ‘hard’. This method is suitable for removing both temporary and permanent types of hardness.
Flux
A mixture of borax and ammonium chloride is used as a flux when welding iron and steel. It lowers the melting point of the unwanted iron oxide (scale), allowing it to run off. Borax is also used mixed with water as a flux when soldering jewelry metals such as gold or silver, where it allows the molten solder to wet the metal and flow evenly into the joint. Borax is also a good flux for "pre-tinning" tungsten with zinc — making the tungsten soft-solderable.[27] Borax is often used as a flux for forge welding.
Small-scale gold mining

Borax is replacing mercury as the preferred method for extracting gold in small-scale mining facilities. The method is called the borax method and is used in the Philippines.[28]
Flubber
A rubbery polymer sometimes called Slime, Flubber, 'gluep' or 'glurch' (or erroneously called Silly Putty, which is based on silicone polymers), can be made by cross-linking polyvinyl alcohol with borax. Making flubber from polyvinyl acetate-based glues, such as Elmer's Glue, and borax is a common elementary-science demonstration.[29][30]
Food additive
Borax, given the E number E285, is used as a food additive, but is banned in some countries, including the United States, China,[31] and Thailand. As a consequence, certain foods, such as caviar, produced for sale in the United States contain higher levels of salt to assist preservation.[32] In addition to its use as a preservative, borax imparts a firm, rubbery texture to food. In China, borax (Chinese: 硼砂; pinyin: péng shā or Chinese: 月石; pinyin: yuè shí) has been found in foods including wheat and rice noodles named; lamian, shahe fen, kway teow, and chee cheong fun.[33] In Indonesia, it is a common, but forbidden, additive to such foods as noodles, bakso (meatballs), and steamed rice. The country's Directorate of Consumer Protection warns of the risk of liver cancer with high consumption over a period of five to ten years.[34] Borax was one of the chemicals used in 19th century industrialised food production that was investigated by Dr. Harvey W. Wiley with his famous 'Poison Squad' as part of the US Department of Agriculture. This led up to the 1906 Pure Food and Drug Act, a landmark event in the early history of food regulation in the United States.
Other uses

Medicinal
- Anti-fungal foot soak
- Treatment for thrush in horses' hoofs
- Is found in some commercial vitamin supplements
Other
- Ingredient in enamel glazes[35]
- Component of glass, pottery, and ceramics[36]
- Used as an additive in ceramic slips and glazes to improve fit on wet, greenware, and bisque
- Fire retardant[37]
- Anti-fungal compound for cellulose insulation[36]
- Moth proofing 10% solution for wool[38]
- Pulverized for the prevention of stubborn pests (e.g. German cockroaches) in closets, pipe and cable inlets, wall panelling gaps, and inaccessible locations where ordinary pesticides are undesirable[39]
- Precursor for sodium perborate monohydrate that is used in detergents, as well as for boric acid and other borates
- Tackifier ingredient in casein, starch and dextrin based adhesives[40]
- Precursor for boric acid, a tackifier ingredient in polyvinyl acetate, polyvinyl alcohol based adhesives
- To make indelible ink for dip pens by dissolving shellac into heated borax[41]
- Curing agent for snake skins
- Curing agent for salmon eggs, for use in sport fishing for salmon
- Swimming pool buffering agent to control pH[42]
- Neutron absorber, used in nuclear reactors and spent fuel pools to control reactivity and to shut down a nuclear chain reaction[43]
- As a micronutrient fertilizer to correct boron-deficient soils.[44][45]
- Preservative in taxidermy[46]
- To color fires with a green tint[47]
- Was traditionally used to coat dry-cured meats such as hams to improve the appearance and discourage flies.[48][49][34]
- For stopping car radiator and engine block leaks
- Used by blacksmiths in forge welding[50]
- Used as a woodworm treatment (diluted in water)
- Used as an insecticide in some ant baits. It kills ants slowly, allowing the ants time to bring the poison back to the colony, killing the queen and eventually the entire colony.
- Deodorizer for carpets, sprinkle on and leave for a while and vacuum it up
Toxicity
Borax, sodium tetraborate decahydrate, according to one study, is not acutely toxic. Its LD50 (median lethal dose) score is tested at 4.55-6.08 g/kg in rats as determined by US EPA (1969),[51] later in 1972 found to be 4.5 g/kg,[52] meaning that a significant dose of the chemical is needed to cause severe symptoms or death. The lethal dose is not necessarily the same for humans. On pesticide information websites it is listed as a non-lethal compound and of no hazardous concerns.
Borax has been in use as an insecticide in the United States with various restrictions since 1946. All restrictions were removed in February 1986 due to the low toxicity of borax, as reported in two EPA documents relating to boric acid and borax.[53][54]
- EPA has determined that, because they are of low toxicity and occur naturally, boric acid and its sodium salts should be exempted from the requirement of a tolerance (maximum residue limit) for all raw agricultural commodities.[53]
Although it cited inconclusive data, a re-evaluation in 2006 by the EPA still found that "There were no signs of toxicity observed during the study and no evidence of cytotoxicity to the target organ."[55] In the reevaluation, a study of toxicity due to overexposure was checked and the findings were that "The residential handler inhalation risks due to boric acid and its sodium salts as active ingredients are not a risk concern and do not exceed the level of concern..." but that there could be some risk of irritation to children inhaling it if used as a powder for cleaning rugs.
Sodium tetraborate decahydrate has no known hazard issues.[56]
Overexposure to borax dust can cause respiratory irritation, while no skin irritation is known to exist due to external borax exposure. Ingestion may cause gastrointestinal distress including nausea, persistent vomiting, abdominal pain, and diarrhea. Effects on the vascular system and human brain include headaches and lethargy, but are less frequent. In severe cases, a "beefy" red rash affecting the palms, soles, buttocks and scrotum has occurred.[57]
Due to concerns about the toxicity of borax which was withdrawn as a cleaning and laundry product, sodium sesquicarbonate is sold in the European Union (EU) as "Borax substitute". It is also known as one of the E number food additives E500.
During the 1951 Indianapolis 500, race winner Lee Wallard coated his racing suit with a fire retardant mixture of borax crystals and water. He suffered serious chafing, and required treatment at the infield hospital after the victory lane celebration.
Risk to fertility and pregnancy
Borax was added to the Substance of Very High Concern (SVHC) candidate list on December 16, 2010. The SVHC candidate list is part of the EU Regulations on the Registration, Evaluation, Authorisation and Restriction of Chemicals 2006 (REACH), and the addition was based on the revised classification of borax as toxic for reproduction category 1B under the CLP Regulations. Substances and mixtures imported into the EU which contain borax are now required to be labelled with the warnings "May damage fertility" and "May damage the unborn child".[58] It was proposed for addition to REACH Annex XIV by the ECHA on July 1, 2015.[59] If this recommendation is approved, all imports and uses of borax in the EU will have to be authorized by the ECHA.
Review of the boron toxicity (as boric acid and borates) published 2012 in Journal of Toxicology and Environmental Health concluded: "It clearly appears that human B [boron] exposures, even in the highest exposed cohorts, are too low to reach the blood (and target tissue) concentrations that would be required to exert adverse effects on reproductive functions."[60]
A draft risk assessment released by Health Canada in July 2016 has found that overexposure to boric acid has the potential to cause developmental and reproductive health effects. Since people are already exposed to boric acid naturally through their diets and water, Health Canada advised that exposure from other sources should be reduced as much as possible, especially for children and pregnant women. The concern is not with any one product, but rather multiple exposures from a variety of sources. With this in mind, the department also announced that registrations for certain pesticides that contain boric acid, which are commonly used in homes, will have their registrations cancelled and be phased out of the marketplace. As well, new, more protective label directions are being introduced for other boric acid pesticides that continue to be registered in Canada (for example, enclosed bait stations and spot treatments using gel formulations).[61]
See also
- Borax bead test
- Sodium sesquicarbonate (Borax Substitute)
- John Veatch
- List of cleaning agents
- Sodium borohydride – Chemical compound
- Ulexite
References
- Haynes, p. 4.91
- Haynes, p. 4.135
- Haynes, p. 4.139
- Levy, H. A.; Lisensky, G. C. (1978). "Crystal structures of sodium sulfate decahydrate (Glauber's salt) and sodium tetraborate decahydrate (borax). Redetermination by neutron diffraction". Acta Crystallographica Section B. 34 (12): 3502–3510. doi:10.1107/S0567740878011504.
- "Potential Commodities NFPA 704" (PDF). Archived from the original (PDF) on May 17, 2016. Retrieved December 9, 2018.
- NIOSH Pocket Guide to Chemical Hazards. "#0057". National Institute for Occupational Safety and Health (NIOSH).
- NIOSH Pocket Guide to Chemical Hazards. "#0059". National Institute for Occupational Safety and Health (NIOSH).
- NIOSH Pocket Guide to Chemical Hazards. "#0058". National Institute for Occupational Safety and Health (NIOSH).
- Nobuo Morimoto (1956): "The crystal structure of borax". Mineralogical Journal, volume 2, issue 1, pages 1-18. doi:10.2465/minerj1953.2.1
- G. J. Gainsford, T. Kemmitt and C. Higham (2008): "Redetermination of the borax structure from laboratory X-ray data at 145 K". Acta Crystallographica Series E (Inorganic Compounds), volume E64, pages i24-i25. doi:10.1107/S1600536808010441
- "March 2012 ipad ewaste Filipino Borax, Pakistans Pollution, Artisanal Gold Mining". Blacksmithinstitute.org. Archived from the original on October 13, 2016. Retrieved August 7, 2016.
- "Borax (Na2B4O7·10H2O ) – Sodium Borate – Occurrence, Discovery and Applications". Amoz.com. August 16, 2004.
- "American Borax Production" Scientific American September 22, 1877
- Hildebrand, G. H. (1982) "Borax Pioneer: Francis Marion Smith." San Diego: Howell-North Books. p. 267 ISBN 0-8310-7148-6
- Mendham, J.; Denney, R. C.; Barnes, J. D.; Thomas, M. J. K. (2000), Vogel's Quantitative Chemical Analysis (6th ed.), New York: Prentice Hall, ISBN 0-582-22628-7 p. 316.
- Staff. "Creating flame colors". The Science Company. Retrieved November 30, 2008.
- Borax decahydrate. borax.com
- "Borax decahydrate (sodium tetraborate decahydrate)".
- "Tincal". Oxford English Dictionary (Online ed.). Oxford University Press. (Subscription or participating institution membership required.)
- Chemical age of India, Vol. 37, No.10 & 11 (1976)
- "Death Valley National Park". nps.gov. National Park Service. Retrieved August 19, 2020.
- Wizniak, Jaime (July 2005). "Borax, Boric Acid, and Boron – From exotic to commodity" (PDF). Indian Journal of Chemical Technology. 12 (4). ISSN 0975-0991.
- Record in the Household Products Database of NLM
- Hammond, C.R. (2004). The Elements, in Handbook of Chemistry and Physics 81st edition. CRC press. ISBN 978-0-8493-0485-9.
- Peacock, Andrew C.; Dingman, C. Wesley (1967). "Resolution of Multiple Ribonucleic Acid Species by Polyacrylamide Gel Electrophoresis". Biochemistry. 6 (6): 1818–1827. doi:10.1021/bi00858a033. PMID 6035921.
- Anderson, S. (1981). "Shotgun DNA sequencing using cloned DNase I-generated fragments". Nucleic Acids Research. 9 (13): 3015–27. doi:10.1093/nar/9.13.3015. PMC 327328. PMID 6269069.
- Dodd, J.G. (1966). "Soft soldering to tungsten wire". American Journal of Physics. 34 (10): xvi. Bibcode:1966AmJPh..34D..16D. doi:10.1119/1.1972398.
- "The borax method" (PDF). Borax replacing mercury in small-scale mining. The Geological Survey of Denmark and Greenland (GEUS). Archived from the original (PDF) on December 6, 2008. Retrieved August 2, 2008.
- Parratore, Phil (1998). Wacky Science: A Cookbook for Elementary Teachers. Dubuque, IA: Kendall Hunt. p. 26. ISBN 978-0-7872-2741-8.
- "Slime Recipe - How to Make Borax and White Glue Slime". Chemistry.about.com. Retrieved August 7, 2016.
- Reiley, Laura (April 22, 2019). "After China turned it into a cheap snack, caviar is at risk of losing its status as a luxury good". The Washington Post. Retrieved April 22, 2019.
- "Caviar glossary". The Caviar Guide a gourmet review of caviars & fish roe. Hanson Ltd, Geneva, Switzerland. Archived from the original on December 8, 2008. Retrieved July 7, 2008.
- "Chinese Ingredients: Borax Powder, Mushroom Extract – Chowhound". Chowhound.chow.com. September 11, 2005. Retrieved August 7, 2016.
- "Watch Out For The Food We Consume". Directorate of Consumer Protection, Jakarta, Indonesia. 2006. Archived from the original on December 28, 2008. Retrieved February 10, 2009.
- "Alphabetical information on pottery glaze making materials and clay body ingredients". Sheffield Pottery. Retrieved December 4, 2019.
- Schubert, David M. (2003). "Borates in Industrial Use". In Roesky, Herbert W.; Atwood, David A. (eds.). Group 13 Chemistry III. Group 13 Chemistry III: Industrial Applications. Structure and Bonding. 105. Springer Berlin Heidelberg. pp. 1–40. doi:10.1007/3-540-46110-8_1. ISBN 978-3-540-46110-4.
- Shen, Kelvin K.; O’Connor, Roderick (1998), Pritchard, Geoffrey (ed.), "Flame retardants: borates", Plastics Additives: An A-Z reference, Polymer Science and Technology Series, Springer Netherlands, pp. 268–276, doi:10.1007/978-94-011-5862-6_30, ISBN 978-94-011-5862-6
- "Centre for Alternative Technology". Cat.org.uk. Archived from the original on August 1, 2012. Retrieved August 7, 2016.
- Murray, Lynda M. (1989). "Least toxic pest control: how infestations of termites, ants, fleas, ticks, and beetles can be controlled without causing short- or long-term indoor air quality changes and health risks". nepis.epa.gov. United States Environmental Protection Agency. Retrieved January 18, 2020.
- Suárez, Juan C. (2011), "Bioadhesives", in da Silva, Lucas F. M.; Öchsner, Andreas; Adams, Robert D. (eds.), Handbook of Adhesion Technology, Springer Berlin Heidelberg, pp. 1385–1408, doi:10.1007/978-3-642-01169-6_53, ISBN 978-3-642-01168-9
- Pfingstag, Gerhard (May 1993). "Colorants in inks for writing, drawing and marking". Journal of the Society of Dyers and Colourists. 109: 188–192.
- , "Buffer System for Swimming Pools and Related Structures", issued 2008-11-17
- "Development of Boron-based materials for nuclear applications" (PDF).
- "Borax". Nature.berkeley.edu. Retrieved August 7, 2016.
- "Boron Basics". www.spectrumanalytic.com.
- Grantz, Gerald J. Home book of taxidermy and tanning. Harrisburg, Pa. ISBN 0-8117-0805-5. OCLC 32505.
- Marie, Anne. "How To Color Fire – Fun Fireplace Instructions". Chemistry.about.com. Retrieved August 7, 2016.
- Nicholls, Walter (November 10, 1991). "THE CUSTOM OF THE COUNTRY HAM". The Washington Post.
- "Report of the State Board of Health of the State of New Hampshire ..., Volume 19". 1906. pp. 169–171.
- Moehring, Jack; Willman, Michael; Pulscher, Isaac; Rowe, Devin (December 2016). "Bladesmithing at South Dakota School of Mines and Technology". JOM. 68 (12): 3186–3192. Bibcode:2016JOM....68l3186M. doi:10.1007/s11837-016-2139-z. ISSN 1047-4838. S2CID 137747858.
- https://www3.epa.gov/pesticides/chem_search/cleared_reviews/csr_PC-011102_1-Aug-69_002.pdf
- Weir, Robert J.; Fisher, Russell S. (November 1, 1972). "Toxicologic studies on borax and boric acid". Toxicology and Applied Pharmacology. 23 (3): 351–364. doi:10.1016/0041-008X(72)90037-3. ISSN 0041-008X. PMID 4673567.
- "Pesticide Reregistration Status | Pesticides | US EPA" (PDF). Epa.gov. Retrieved August 7, 2016.
- "Pesticides | US EPA" (PDF). Epa.gov. August 20, 2015. Retrieved August 7, 2016.
- "Archived copy". Archived from the original on May 3, 2015. Retrieved April 27, 2015.CS1 maint: archived copy as title (link)
- "Safety Data Sheet : acc. to OSHA HCS" (PDF). Aquasolutions.org. Retrieved August 7, 2016.
- Reigart, J. Routt (2009). Recognition and Management of Pesticide Poisonings (5th Ed. ). DIANE Publishing. p. 76. ISBN 978-1-4379-1452-8. Retrieved June 4, 2020.
- Member state committee draft support document for identification of disodium tetraborate, anhydrous as a substance of very high concern because of its CMR properties. Adopted on June 9, 2010. Echa.europa.eu. Retrieved on February 17, 2012.
- Recommendation of the European Chemicals Agency of 1 July 2015 for the inclusion of substances in Annex XIV to REACH (List of Substances subject to Authorisation) Echa.europa.eu. Retrieved on July 6, 2015.
- Bolt, Hermann M.; Başaran, Nurşen; Duydu, Yalçın (2012). "Human Environmental and Occupational Exposures to Boric Acid: Reconciliation with Experimental Reproductive Toxicity Data". Journal of Toxicology and Environmental Health, Part A. 75 (8–10): 508–514. doi:10.1080/15287394.2012.675301. PMID 22686310. S2CID 31972554.
- "Information Update – Health Canada advises Canadians to avoid homemade craft and pesticide recipes using boric acid – Recalls & alerts – Healthy Canadians Website". Healthycanadians.gc.ca. July 22, 2016. Retrieved August 7, 2016.
Cited sources
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. ISBN 978-1439855119.
External links
| Wikimedia Commons has media related to Borax. |
| Look up borax in Wiktionary, the free dictionary. |
| Wikisource has the text of the 1879 American Cyclopædia article Borax. |
