Sodium silicate
Sodium silicate is a generic name for chemical compounds with the formula Na
2xSi
yO
2y+x or (Na
2O)
x·(SiO
2)
y, such as sodium metasilicate Na
2SiO
3, sodium orthosilicate Na
4SiO
4, and sodium pyrosilicate Na
6Si
2O
7. The anions are often polymeric. These compounds are generally colorless transparent solids or white powders, and soluble in water in various amounts.
Sodium silicate is also the technical and common name for a mixture of such compounds, chiefly the metasilicate, also called waterglass, water glass, or liquid glass. The product has a wide variety of uses, including the formulation of cements, passive fire protection, textile and lumber processing, manufacture of refractory ceramics, as adhesives, and in the production of silica gel. The commercial product, available in water solution or in solid form, is often greenish or blue owing to the presence of iron-containing impurities.
In industry, the various grades of sodium silicate are characterized by their SiO2:Na2O weight ratio (which can be converted to molar ratio by multiplication with 1.032). The ratio can vary between 1:2 and 3.75:1.[1] Grades with ratio below 2.85:1 are termed alkaline. Those with a higher SiO2:Na2O ratio are described as neutral.
History
Soluble silicates of alkali metals (sodium or potassium) were observed by European alchemists already in the 1500s. Giambattista della Porta observed in 1567 that tartari salis (cream of tartar, potassium hydrogen tartrate) caused powdered crystallum (quartz) to melt at a lower temperature.[2] Other possible early references to alkali silicates were made by Basil Valentine in 1520,[3] and by Agricola in 1550. Around 1640, Jean Baptist van Helmont reported the formation of alkali silicates as a soluble substance made by melting sand with excess alkali, and observed that the silica could be precipitated quantitatively by adding acid to the solution.[4]
In 1646, Glauber made potassium silicate, that he termed liquor silicum by melting potassium carbonate (obtained by calcinating cream of tartar) and sand in a crucible, and keeping it molten until it ceased to bubble (due to the release of carbon dioxide). The mixture was allowed to cool and then was ground to a fine powder. When the powder was exposed to moist air, it gradually formed a viscous liquid, which Glauber called "Oleum oder Liquor Silicum, Arenæ, vel Crystallorum" (i.e., oil or solution of silica, sand or quartz crystal).[5][6]
However, it was later claimed that the substances prepared by those alchemists were not waterglass as it is understood today.[7][8][9] That would have been prepared in 1818 by Johann Nepomuk von Fuchs, by treating silicic acid with an alkali; the result being soluble in water, "but not affected by atmospheric changes".[10][11]
The terms "water glass" and "soluble glass" were used by Leopold Wolff in 1846,[12] by Émile Kopp in 1857,[13] and by Hermann Krätzer in 1887.[14]
In 1892, Rudolf Von Wagner distinguished soda, potash, double (soda and potash), and fixing (i.e., stabilizing) as types of water glass. The fixing type was "a mixture of silica well saturated with potash water glass and a sodium silicate" used to stabilize inorganic water color pigments on cement work for outdoor signs and murals.[15][16][17][18]
Properties
Sodium silicates are colorless glassy or crystalline solids, or white powders. Except for the most silicon-rich ones, they are readily soluble in water, producing alkaline solutions.
Sodium silicates are stable in neutral and alkaline solutions. In acidic solutions, the silicate ions react with hydrogen ions to form silicic acids, which tend to decompose into hydrated silicon[19] dioxide gel. Heated to drive off the water, the result is a hard translucent substance called silica gel, widely used as a desiccant. It can withstand temperatures up to 1100 °C.
Production
Solutions of sodium silicates can be produced by treating a mixture of silica (usually as quartz sand), caustic soda, and water, with hot steam in a reactor. The overall reaction is
- 2x NaOH + SiO
2 → (Na
2O)
x·SiO
2 + x H
2O
Sodium silicates can also be obtained by dissolving silica SiO
2 (whose melting point is 1713 °C) in molten sodium carbonate (that melts with decomposition at 851 °C):[20]
- x Na
2CO
3 + SiO
2 → (Na
2O)
x·SiO
2 + CO
2
The material can be obtained also from sodium sulfate (melting point 884 °C) with carbon as a reducing agent:
- 2x Na
2SO
4 + C + 2 SiO
2 → 2 (Na
2O)
x·SiO
2 + 2 SO
2 + CO
2
In 1990, 4 million tons of alkali metal silicates were produced.[1]
Uses
The main applications of sodium silicates are in detergents, paper, water treatment, and construction materials.[1]
Adhesive
The largest application of sodium silicate solutions is a cement for producing cardboard.[1] When used as a paper cement, the tendency is for the sodium silicate joint eventually to crack within a few years, at which point it no longer holds the paper surfaces cemented together.
Drilling fluids
Sodium silicate is frequently used in drilling fluids to stabilize borehole walls and to avoid the collapse of bore walls. It is particularly useful when drill holes pass through argillaceous formations containing swelling clay minerals such as smectite or montmorillonite.
Concrete and general masonry treatment
Concrete treated with a sodium silicate solution helps to reduce porosity in most masonry products such as concrete, stucco, and plasters. This effect aids in reducing water penetration, but has no known effect on reducing water vapor transmission and emission.[21] A chemical reaction occurs with the excess Ca(OH)2 (portlandite) present in the concrete that permanently binds the silicates with the surface, making them far more durable and water repellent. This treatment generally is applied only after the initial cure has taken place (7 days or so depending on conditions). These coatings are known as silicate mineral paint.
Detergent auxiliaries
It is used in detergent auxiliaries such as complex sodium disilicate and modified sodium disilicate. The detergent granules gain their ruggedness from a coating of silicates.[1]
Water treatment
Sodium silicate is used as an alum coagulant and an iron flocculant in wastewater treatment plants. Sodium silicate binds to colloidal molecules, creating larger aggregates that sink to the bottom of the water column. The microscopic negatively charged particles suspended in water interact with sodium silicate. Their electrical double layer collapses due to the increase of ionic strength caused by the addition of sodium silicate (doubly negatively charged anion accompanied by two sodium cations) and they subsequently aggregate. This process is called coagulation.[1]
Refractory use
Water glass is a useful binder of solids, such as vermiculite and perlite. When blended with the aforementioned lightweight aggregates, water glass can be used to make hard, high-temperature insulation boards used for refractories, passive fire protection and high temperature insulations, such as moulded pipe insulation applications. When mixed with finely divided mineral powders, such as vermiculite dust (which is common scrap from the exfoliation process), one can produce high temperature adhesives. The intumescence disappears in the presence of finely divided mineral dust, whereby the waterglass becomes a mere matrix. Waterglass is inexpensive and abundantly available, which makes its use popular in many refractory applications.
Sand casting
It is used as a binder of the sand when doing sand casting of iron or steel. It allows the rapid production of a strong mold, by passing CO2 through the mixture of sand and sodium silicate in the mold box, which hardens it almost instantly.
Dye auxiliary
Sodium silicate solution is used as a fixative for hand dyeing with reactive dyes that require a high pH to react with the textile fiber. After the dye is applied to a cellulose-based fabric, such as cotton or rayon, or onto silk, it is allowed to dry, after which the sodium silicate is painted on to the dyed fabric, covered with plastic to retain moisture, and left to react for an hour at room temperature.[22]
Passive fire protection
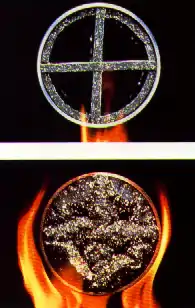

Sodium silicates are inherently intumescent. They come in prill (solid beads) form, as well as the liquid, water glass. The solid sheet form (Palusol) must be waterproofed to ensure long-term passive fire protection (PFP).
Standard, solid, bead-form sodium silicates have been used as aggregate within silicone rubber to manufacture plastic pipe firestop devices. The silicone rubber was insufficient waterproofing to preserve the intumescing function and the products had to be recalled, which is problematic for firestops concealed behind drywall in buildings.
Pastes for caulking purposes are similarly unstable. This, too, has resulted in recalls and even litigation. Only 3M's "Expantrol" version, which has an external heat treatment that helps to seal the outer surface, as part of its process standard, has achieved sufficient longevity to qualify for DIBt approvals in the US for use in firestopping.
Not unlike other intumescents, sodium silicate, both in bead form and in liquid form, are inherently endothermic, due to liquid water in the water glass and hydrates in the prill form. The absence in the US of mandatory aging tests, whereby PFP systems are made to undergo system performance tests after the aging and humidity exposures, are at the root of the continued availability, in North America, of PFP products that can become inoperable within weeks of installation. Indiscriminate use of sodium silicates without proper waterproofing measures are contributors to the problems and risk. When sodium silicates are adequately protected, they function extremely well and reliably for long periods. Evidence of this can be seen in the many DIBt approvals for plastic pipe firestop devices using Palusol (a product of BASF), which use waterproofed sodium silicate sheets.
Metal repair
Sodium silicate is used, along with magnesium silicate, in muffler repair and fitting paste. When dissolved in water, both sodium silicate and magnesium silicate form a thick paste that is easy to apply. When the exhaust system of an internal combustion engine heats up to its operating temperature, the heat drives out all of the excess water from the paste. The silicate compounds that are left over have glass-like properties, making a temporary, brittle repair.
Automotive repair
Sodium silicate is also used currently as an exhaust system joint and crack sealer for repairing mufflers, resonators, tailpipes, and other exhaust components, with and without fiberglass reinforcing tapes. In this application, the sodium silicate (60–70%) is typically mixed with kaolin (40-30%), an aluminium silicate mineral, to make the sodium silicate "glued" joint opaque. The sodium silicate, however, is the high-temperature adhesive; the kaolin serves simply as a compatible high-temperature coloring agent. Some of these repair compounds also contain glass fibres to enhance their gap-filling abilities and reduce brittleness.
Sodium silicate can be used to fill gaps within the head gasket. Commonly used on aluminum alloy cylinder heads, which are sensitive to thermally induced surface deflection. This can be caused by many things including head-bolt stretching, deficient coolant delivery, high cylinder head pressure, overheating, etc.
"Liquid glass" (sodium silicate) is added to the system through the radiator, and allowed to circulate. Sodium silicate is suspended in the coolant until it reaches the cylinder head. At 100–105 °C (212-221 °F), sodium silicate loses water molecules to form a glass seal with a remelt temperature above 810 °C (1,490 °F).
A sodium silicate repair can last two years or longer. The repair occurs rapidly, and symptoms disappear instantly. This repair works only when the sodium silicate reaches its "conversion" temperature at 100–105 °C. Contamination of engine oil is a serious possibility in situations in which a coolant-to-oil leak is present. Sodium silicate (glass particulate) contamination of lubricants is detrimental to their function.
Sodium silicate solution is used to inexpensively, quickly, and permanently disable automobile engines. Running an engine with about 2 liters of a sodium silicate solution instead of motor oil causes the solution to precipitate, catastrophically damaging the engine's bearings and pistons within a few minutes.[23] In the United States, this procedure was used to comply with requirements of the Car Allowance Rebate System (CARS) program.[23][24]
Safe construction
A mixture of sodium silicate and sawdust has been used in between the double skin of certain safes. This not only makes them more fire resistant, but also makes cutting them open with an oxyacetylene torch extremely difficult due to the smoke emitted.
Crystal gardens
When crystals of a number of metallic salts are dropped into a solution of water glass, simple or branching stalagmites of coloured metal silicates are formed. This phenomenon has been used by manufacturers of toys and chemistry sets to provide instructive enjoyment to many generations of children from the early 20th century until the present. An early mention of crystals of metallic salts forming a "chemical garden" in sodium silicate is found in the 1946 Modern Mechanix magazine.[25] Metal salts used included the sulfates and/or chlorides of copper, cobalt, iron, nickel, and manganese.
Pottery
Sodium silicate is used as a deflocculant in casting slips helping reduce viscosity and the need for large amounts of water to liquidize the clay body. It is also used to create a crackle effect in pottery, usually wheel-thrown. A vase or bottle is thrown on the wheel, fairly narrow and with thick walls. Sodium silicate is brushed on a section of the piece. After 5 minutes, the wall of the piece is stretched outward with a rib or hand. The result is a wrinkled or cracked look.
It is also the main agent in "magic water", which is used when joining clay pieces, especially if the moisture level of the two differs.[26]
Sealing of leaking water-containing structures
Sodium silicate with additives was injected into the ground to harden it and thereby to prevent further leakage of highly radioactive water from the Fukushima Daiichi nuclear power plant in Japan in April, 2011.[27] The residual heat carried by the water used for cooling the damaged reactors accelerated the setting of the injected mixture.
On June 3, 1958, the USS Nautilus, the world's first nuclear submarine, visited Everett and Seattle. In Seattle, crewmen dressed in civilian clothing were sent in to secretly buy 140 quarts of an automotive product containing sodium silicate (originally identified as Stop Leak) to repair a leaking condenser system. The Nautilus was en route to the North Pole on a top secret mission to cross the North Pole submerged.[28]
Firearm cartridges
A historical use of the adhesive properties of sodium silicates is the production of paper cartridges for black powder revolvers produced by Colt's Manufacturing Company during the period from 1851 until 1873, especially during the American Civil War. Sodium silicate was used to seal combustible nitrated paper together to form a conical paper cartridge to hold the black powder, as well as to cement the lead ball or conical bullet into the open end of the paper cartridge. Such sodium silicate cemented paper cartridges were inserted into the cylinders of revolvers, thereby speeding the reloading of cap-and-ball black powder revolvers. This use largely ended with the introduction of Colt revolvers employing brass-cased cartridges starting in 1873.[29][30] Similarly, sodium silicate was also used to cement the top wad into brass shotgun shells, thereby eliminating any need for a crimp at the top of the brass shotgun shell to hold a shotgun shell together. Reloading brass shotgun shells was widely practiced by self-reliant American farmers during the 1870s, using the same waterglass material that was also used to preserve eggs. The cementing of the top wad on a shotgun shell consisted of applying from three to five drops of waterglass on the top wad to secure it to the brass hull. Brass hulls for shotgun shells were superseded by paper hulls starting around 1877. The newer paper-hulled shotgun shells used a roll crimp in place of a waterglass-cemented joint to hold the top wad in the shell. However, whereas brass shotshells with top wads cemented with waterglass could be reloaded nearly indefinitely (given powder, wad, and shot, of course), the paper hulls that replaced the brass hulls could be reloaded only a few times.
Food and medicine
While not actually a medical use, sodium silicate, and other silicates, are the primary components in "instant" wrinkle remover creams, which temporarily tighten the skin to minimize the appearance of wrinkles & under-eye bags. These creams, when applied as a thin film and allowed to dry for a few minutes, can present dramatic results. Unfortunately, it is not permanent, lasts few minutes or couple of hours. It works like water cement, once the muscle starts to move, it cracks and leaves white residues on the skin.
Food preservation

Waterglass has been used as an egg preservative with large success, primarily when refrigeration is not available. Fresh-laid eggs are immersed in a solution of sodium silicate (waterglass). After being immersed in the solution they were removed and allowed to dry. A permanent air tight coating remains on the eggs. If they are then stored in appropriate environment, the majority of bacteria which would otherwise cause them to spoil are kept out and their moisture is kept in. According to the cited source, treated eggs can be kept fresh using this method for up to five months. When boiling eggs so preserved, the shell is no longer permeable to air, and the egg will tend to crack unless a hole in the shell is made (e.g. with a pin) in order to allow steam to escape.[31]
Homebrewing
Sodium silicate flocculant properties are also used to clarify wine and beer by precipitating colloidal particles. As a clearing agent, though, sodium silicate is sometimes confused with isinglass which is prepared from collagen extracted from the dried swim bladders of sturgeon and other fishes. Eggs preserved in a bucket of waterglass gel, and their shells are sometimes also used (baked and crushed) to clear wine.[32]
Aquaculture
Sodium silicate gel is also used as a substrate for algal growth in aquaculture hatcheries.[33]
See also
References
- Gerard Lagaly, Werner Tufar, A. Minihan, A. Lovell "Silicates" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, 2005. doi:10.1002/14356007.a23_661
- Giambattista della Porta (1569): Magia naturalis sive de miraculis rerum naturalium, libri iiii (Natural magic or on the miracles of nature, in four books); pages 290–291, "Crystallus, ut fusilis fiat" (quartz, so made molten). Published by Guillaume Rouillé (Gulielmum Rovillium) in Lyon (Lugdunum) France
- Kohn, C. (1862): "Die Erfindung des Wasserglas im Jahre 1520" (The invention of waterglass in the year 1520), Zeitschrift des Oesterreichischen Ingenieur-Vereins (Journal of the Austrian Engineer Association), volume 14, pages 229–230.
- Johannes van Helmont (1644): Opuscula medica inaudita, published by Jost Kalckhoven (Jodocum Kalcoven) Cologne, Germany. In Part I: De Lithiasi, page 53, van Helmont mentions that alkalis dissolve silicates: "Porro lapides, gemmae, arenae, marmora, silices, &c. adjuncto alcali, vitrificantur: sin autem plure alcali coquantur, resolvuntur in humido quidem: ac resoluta, facili negotio acidorum spirituum, separantur ab alcali, pondere pristini pulveris lapidum." (Furthermore, stone, gems, sand, marble, silica, etc., become glassy by the addition of alkali: but if roasted with more alkali, they are dissolved in moisture: and the former weight of the stone powder is separated from the alkali and released by simply adding acid.)
- Johann Rudolf Glauber (1646), Furni Novi Philosophici (New Philosophical Furnace). Published by Johan Jansson, Amsterdam.
- Johann Rudolf Glauber (1661): Furni Novi Philosophici Oder Beschreibung einer New-erfundenen Distillir-Kunst (New Philosophical Furnace, or Treatise on Newly Discovered Distillation Art) chapter LXXIX, pages 164–166: "Wie durch Hülff eines reinen Sandes oder Kißlings auß Sale Tartari ein kräfftiger Spiritus kan erlanget werden." (How with the help of a pure sand or silica a powerful solution can be gotten from cream of tartar).
- Anon. (1863): "Die Erfindung des Wasserglases im Jahre 1520." Kunst- und Gewerbe-Blatt, volume 49, pages 228–230.
- Anon. (1863): "Die Erfindung des Wasserglases im Jahre 1520." Reprint, Polytechnisches Journal, volume 168, pages 394–395
- Anon. (1863) "Die angebliche Erfindung des Wasserglases im Jahre 1520" (On the alleged invention of waterglass in the year 1520). Reprint, Neues Repertorium für Pharmacie, volume 12, pages 271–273.
- Johann Nepomuk von Fuchs (1825) "Ueber ein neues Produkt aus Kieselerde und Kali" (On a new product from silica and potash), Archiv für die gesammte Naturlehre, volume 5, issue 4, pages 385–412. On page 386: "Ich erhielt es zuerst, vor ungefähr 7 Jahren" (I first obtained it about 7 years ago).
- Joh. Nepomuk Fuchs (1825) "Ueber ein neues Produkt aus Kieselerde und Kali; und dessen nüzliche Anwendung als Schuzmittel gegen schnelle Verbreitung des Feuers in Theatern, als Bindemittel, firnißartigen Anstrichen u.s.w." (On a new product from silica and potash; and its useful application as a protection against the rapid spread of fire in theaters, as a glue, varnish, etc.). Polytechnisches Journal, volume 17, pages 465–481.
- Leopold Wolff (1846): Das Wasserglas: Seine Darstellung, Eigenschaften und seine mannichfache Anwendung in den technischen Gewerben (Water-glass: its preparation, properties, and its manifold uses in technical commerce) published by Quedlinburg, Leipzig, Germany.
- Emile Kopp (1857): "Sur la préparation et les propriétés du verre soluble ou des silicates de potasse et de soude; analyse de tous les travaux publiés jusqu'a ce jour sur ce sujet" (On the preparation and properties of soluble glass or the silicates of potash and soda; analysis of all works published until today on this subject). Le Moniteur scientifique, volume 1, 337–349, pages 366–391.
- Hermann Krätzer (1887): Wasserglas und Infusorienerde, deren Natur und Bedeutung für Industrie, Technik und die Gewerbe (Water-glass and soluble earths, their nature and significance for industry, technology, and commerce). Published by Hartleben, Vienna, Austria.
- Von Wagner, Rudolf (1892; translation of 13th edition by Willian Crookes) Manual of Chemical Technology
- Von Wagner, Manual of Chemical Technology (1892 translation)
- Hermann Mayer (1925): Das Wasserglas; Sein Eigenschaften, Fabrikation und Verwendung auf Grund von Erfahrungen und Mitteilungen der Firma Henkel & Cie. (The Water-glass: Its properties, production, and application on the basis of experiences and communications of the firm of Henkel & Co.) Published by Vieweg, Braunschweig, Germany.
- Morris Schrero (1922): Water-glass: A Bibliography. Published by Carnegie Library, Pittsburgh, Pennsylvania.
- Christopher Gelpi; Peter Feaver; Jason Reifler (2005). Replication data for: Success Matters: Casualty Sensitivity and the War in Iraq. OCLC 795918959.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- http://www.sgh.com/sites/default/files/downloads/technicalbrief_alkali_silicate_based_admixtures.pdf
- Burch, Paula (March 22, 2010). "Sodium silicate as a fixative for dyeing". Retrieved March 22, 2010.
- Helliker, Kevin. "The Killer App for Clunkers Breathes Fresh Life Into 'Liquid Glass'" The Wall Street Journal, 4 August 2009.
- Engine Disablement Procedures for the CARS program Archived 2010-10-19 at the Wayback Machine, cars.gov
- "Magic garden". Mechanix Illustrated: 88. April 1946.
- http://lakesidepottery.com/HTML%20Text/Tips/pottery-magic-mud-magic-water-paper-clay.htm
- Press Release (TEPCO:2011.4.6)
- Commander William R. Anderson with Clay Blair Jr., Nautilus 90 North (Cleveland and New York: The World Publishing Co., 1959), pp. 133–137; Commander William R. Anderson with Clay Blair Jr., Nautilus 90 North (New York: The New American Library, 1959), 89–90
- Tom Kelley (August 1995). "Making and using combustible paper pistol cartridges".
- Kirst, W.J. (1983). Self consuming paper cartridges for the percussion revolver. Minneapolis, Minnesota: Northwest Development Co.
- How To Store Fresh Eggs. motherearthnews.com
- SM Tritton (1956) Amateur wine making.
- Bechtold, M. F. (1955). "Polymerization and Properties of Dilute Aqueous Silicic Acid from Cation Exchange". The Journal of Physical Chemistry. 59 (6): 532–541. doi:10.1021/j150528a013.
Further reading
- Ashford's Dictionary of Industrial Chemicals, third edition, 2011, page 8369.
External links
| Wikimedia Commons has media related to Sodium silicates. |
- Centre Européen d'Etudes des Silicates
- International Chemical Safety Card 1137
- ChemSub Online : Silicic acid, sodium salt
- ChemSub Online : Sodium metasilicate