Bowfin
Bowfin (Amia calva) are bony fish related to gars in the infraclass Holostei. Common names include mudfish, mud pike, dogfish, griddle, grinnel, swamp trout, and choupique. They are regarded as taxonomic relicts, being the sole surviving species of the order Amiiformes, which dates from the Jurassic to the Eocene, persisting to the present. Although bowfin are highly evolved, they are often referred to as "primitive fish" because they have retained some morphological characteristics of their early ancestors.
| Bowfin | |
|---|---|
 | |
| Bowfin in aquarium | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Actinopterygii |
| Order: | Amiiformes |
| Family: | Amiidae |
| Genus: | Amia Linnaeus, 1766 |
| Species: | A. calva |
| Binomial name | |
| Amia calva Linnaeus, 1766 | |
| Synonyms[2][3] | |
|
Genus
Species
| |
Bowfin are demersal freshwater piscivores native to North America, and commonly found throughout much of the eastern United States, and in southern Ontario and Quebec. Fossil deposits indicate Amiiformes were once widespread in both freshwater and marine environments with a range that spanned across North and South America, Europe, Asia and Africa. Now their range is limited to much of the eastern United States and adjacent southern Canada, including the drainage basins of the Mississippi River, Great Lakes and various rivers exiting in the Eastern Seaboard or Gulf of Mexico. Their preferred habitat includes vegetated sloughs, lowland rivers and lakes, swamps and backwater areas; they are also occasionally found in brackish water. They are stalking, ambush predators known to move into the shallows at night to prey on fish and aquatic invertebrates such as crawfish, mollusks, and aquatic insects.
Like gars, bowfin are bimodal breathers which means they have the capacity to breathe both water and air. Their gills exchange gases in the water allowing them to exploit oxygen for breathing, but they also have a gas bladder that serves to maintain buoyancy, and also allows them to breathe air by means of a small pneumatic duct connected from the foregut to the gas bladder. They can break the surface to gulp air, which allows them to survive conditions of aquatic hypoxia that would be lethal to most other species.
Morphology
The average length of a bowfin is 50 cm (20 in);[4] females typically grow to 65–70 cm (26–28 in), males to 50–65 cm (20–26 in).[5] Records indicate bowfin can reach 109 cm (43 in) in length, and weigh 9.75 kg (21.5 lb).[6] Young of the year typically grow to 13–23 cm (5.1–9.1 in) by October.[7] Females tend to grow larger than males.[8][9]

The body of the bowfin is elongated and cylindrical, with the sides and back olive to brown in color, often with vertical bars, and dark reticulations, or camouflaged pattern. The dorsal fin has horizontal bars, and the caudal fin has irregular vertical bars. The underside is white or cream, and the paired fins and anal fin are bright green. During larval stage, hatchlings from about 7–10 mm (0.28–0.39 in) total length are black and tadpole-like in appearance.[10] At approximately 25 mm (0.98 in) total length they have been described as looking like miniature placoderms.[11] They grow quickly, and typically leave the nest within 4 to 6 weeks after hatching.[12] Young males have a black eyespot on the base of the tail (caudal peduncle) that is commonly encircled by an orange-yellowish border while the female's is black, if present at all. It is thought the purpose of the eyespot is to confuse predators, deflecting attacks away from the head of the fish to its tail, which affords the bowfin an opportunity to escape predation.[7][13][14] The bowfin is so named for its long, undulating dorsal fin consisting of 145 to 250 rays, and running from the middle of the back to the base of the tail.
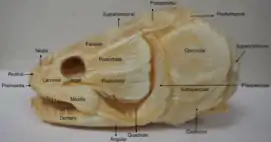
The skull of the bowfin is made of two layers of skull, the dermatocranium and the chondrocranium. The chondrocranium layer cannot be seen because it is located below the dermal bones. The bowfin skull is made up of 28 fused bones, which compose the dermatocranium. The roof of the mouth is made up of 3 bones, the ectopterygoid, the palantine, and the vomer. The teeth are on two bones, the premaxillae and the maxillae. Another three bones make up the lower jaw the dentary, the angular, and the surangular. The cranial surface of the skull is made up of the nasals, the antorbital, the lacrimal, the parietal, the intertemporal, the post parietal, the supratemporal, the extra scapular, the post temporal, and the opercular. The entirety of the skull is attached to the girdle through another set of bones.[15]

Bowfin are often referred to as "living fossils", or "primitive fish" because they retained some of the primitive characters common to their ancestral predecessors,[16] including a modified (rounded externally) heterocercal caudal fin, a highly vascularized gas bladder lung, vestiges of a spiral valve, and a bony gular plate.[16][17] The bony gular plate is located underneath the head on the exterior of the lower jaw between the two sides of the lower jaw bone. Other distinguishing characteristics include long, sharp teeth, and two protruding tube-like nostrils.[13] Unlike all of the most primitive actinopterygians, the scales of bowfin differ in that they are not ganoid scales, rather they are large, single-layered cycloid scales closer in similarity to more derived teleosts.[18][19]
Fish similar in appearance
Northern snakeheads (Channa argus) are commonly mistaken for bowfin because of similarities in appearance, most noticeably their elongated, cylindrical shape, and long dorsal fin that runs along their backs. Northern snakeheads are piscivorous fish native to the rivers and estuaries of China, Russia, and Korea.[20] However, unlike bowfin which are native to North America, the northern snakehead is considered an invasive species and environmentally harmful.[21][22] Some contrasting differences in bowfin include a black eyespot on their caudal peduncle, a tan and olive coloration, a shorter anal fin, a more rounded head, and an upper jaw that is longer than its lower jaw.[23]
The burbot, a predatory fish native to streams and lakes of North America and Eurasia, is also commonly mistaken for bowfin. Burbots can be distinguished by their flat head and chin barbel, long anal fin, and pelvic fins situated beneath the pectoral fins.[24]
Evolution of bowfin morphology
The first fish lacked jaws and used negative pressure to suck their food in through their mouths. The jaw in the bowfin is a result of their evolutionary need to be able to catch and eat bigger and more nutritious prey. As a result of being able to gather more nutrients, Bowfin are able to live a more active lifestyle. The jaw of a bowfin has several contributions. The maxilla and premaxilla are fused and the posterior chondrocranium articulates with the vertebra which allows the jaw freedom to rotate. The suspensorium includes several bones and articulates with the snout, brain case, and the mandible. When the jaw opens epaxial muscles lift the chondrocranium which is attached to the upper jaw, and adductor muscles close the lower jaw. This ability to open and close the jaw allows the bowfin to become more of a predator, in that it can catch bigger prey and be able to mechanical catch, and digest it.[25]
The vertebral column in bowfin is ossified and in comparison to earlier fish, the centra are the major support for the body, whereas in earlier fish the notochord was the main form of support. Neural spines and ribs provide additional support and help stabilize unpaired fins. In bowfin neural spines and ribs also increase in prominence, an evolutionary aspect that helps them stabilize unpaired fins. The evolution of the vertebral column allows the bowfin to withstand lateral bending that puts the column under compression without breaking. This, in turn, allows the bowfin to have more controlled and powerful movements, in comparison to fish that had only a notochord. The bowfin has a rounded heterocercal tail that resembles a homocercal tail. This type of tail gives the body a streamlined shape which allows the bowfin to improve its swimming ability by reducing drag. These types of tails are common in fish with gas bladders, because the bladder supplies the fish with natural buoyancy.[25]

The bowfin is an actinopterygii which means that the pectoral girdle is partly endochondral but mostly dermal bone. In this group of fish the fins function to maneuver, brake, and for slight positional adjustments. The pectoral girdle of the bowfin has six parts. The post temporal, supracleithrum, postcleithrum, cleithrum, scapulacoracoid, and the clavicle make up the pectoral girdle. The pectoral girdle is attached to the skull. The pectoral girdle is made of mostly dermal and some endochondral bone. The paired pectoral and pelvic fins of fish are homologous with the limbs of tetrapods.[25]
Physiology

Bowfin, like other physostomes such as bichirs (Polypteridae), gars (Lepisosteidae), and the lungfish (Dipnoi), are capable of bimodal respiration. They can extract oxygen from the water when breathing through their gills, and can also break the water's surface to breathe or gulp air through a small pneumatic duct connected from their foregut to the gas bladder.[26][27] When performing low-level physical activity, bowfin obtain more than half of their oxygen from breathing air.[28] Bowfin have two distinct air-breathing mechanisms used to ventilate the gas bladder. Type I air breaths are consistent with the action of exhale-inhale stimulated by aerial or aquatic hypoxia to regulate O2 gas exchange; type II air breaths are by inhalation alone which is believed to regulate gas bladder volume for buoyancy control.[27] Bimodal respiration helps bowfin survive and maintain their metabolic rate in hypoxic conditions.[29][30][31] The rate of air breathing is higher in darkness, when the fish is more active.[4]
Bowfin blood can adapt to warm, acidic waters.[7] The fish becomes inactive in waters below 10 °C (50 °F);[7] at this temperature they breathe almost no air; however, with increasing temperature their air breathing increases.[4] Their preferred temperature range is between 12–26 °C (54–79 °F), with 18 °C (64 °F) the temperature of maximum activity.[32] Air breathing is at a maximum in the range 18.4–29.6 °C (65.1–85.3 °F).
Herpetologist Wilfred T. Neill reported in 1950 that he unearthed a bowfin aestivating in a chamber 4 inches (10 cm) below the ground surface, 8 inches (20 cm) in diameter, .25 miles (0.4 km) from a river. It was further noted that flood levels had previously reached the area, and receded. It is not unusual for riverine species like bowfin to move into backwaters with flood currents, and become trapped when water levels recede.[4][33][34][35] While aestivation is anecdotally documented by multiple researchers, laboratory experiments have suggested instead that bowfin are physiologically incapable of surviving more than three to five days of air exposure. However, no field manipulation has been performed.[36][37] Regardless of the lack of evidence confirming the bowfin's ability to aestivate, it has been noted that bowfin can survive prolonged conditions of exposure to air because they have the ability to breathe air. Their gill filaments and lamellae are rigid in structure which helps prevent the lamellae from collapsing and aids gas exchange even during air exposure.[38]
Evolution and phylogeny
Competing hypotheses and debates continue over the evolution of Amia and relatives, including their relationship among basal extant teleosts, and organization of clades.[39] Bowfin are the last remaining member of Halecomorphi, a group that includes many extinct species in several families.[40] Halecomorphs were generally accepted as the sister group to Teleostei but not without question. While a halecostome pattern of neopterygian clades was produced in morphology-based analyses of extant actinopterygians, a different result was produced with fossil taxa which showed a monophyletic Holostei. Monophyletic Holostei were also recovered by at least two nuclear gene analyses, in an independent study of fossil and extant fish,[41][42] and in an analysis of ultraconserved genomic elements.[43]
The extant ray-finned fish of the subclass Actinopterygii include 42 orders, 431 families and over 23,000 species.[44] They are currently classified into two infraclasses, Chondrostei (holosteans) and Neopterygii (teleost fishes).[45] Sturgeons, paddlefish, bichirs and reed fish compose the thirty-eight species of chondrosteans, and are considered relict species. Included in the over 23,000 species of neopterygians are eight relict species comprising gars and the bowfin.[44]
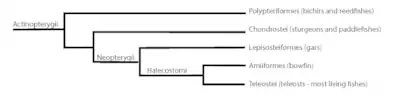
Infraclass Neopterygii
Neopterygians are the second major occurrence in the evolution of ray-finned fish and today include the majority of modern bony fish.[44] They are distinguished from their earlier ancestors by major changes to the jaws, shape of the skull, and tail. They are divided into three divisions:
- Division 1. Order Lepisosteiformes – the relict gars which include extant species of gars that first appeared in the Cretaceous.
- Division 2. Order Amiiformes – the relict bowfin, (halecomorphids), the only extant species in the order Amiiformes which date back to the Triassic period.
- Division 3. Division Teleostei – the stem group of Teleostei from which modern fish arose, including most of the bony fish we are familiar with today.[46]
Species
List of species.[47]
- †Amia depressa Marsh 1871
- †Amia dictyocephala Cope 1875
- †Amia elegans Leidy 1873
- †Amia exilis Lambe 1908
- †Amia fragosa (Jordan 1927)
- †Amia godai Yabumoto & Grande 2013
- †Amia gracilis Leidy 1873
- †Amia lewesiensis Mantell 1822
- †Amia macrospondyla Cope 1891
- †Amia media Leidy 1873
- †Amia morini Priem 1911
- †Amia newberriana Marsh 1871
- †Amia selwyniana Ami 1891
- †Amia uintaensis Leidy 1873
- †Amia whiteavesiana Cope 1891
- †Amia pattersoni
- †Amia scutata Cope 1875
- Amia calva Linnaeus 1766 (Bowfin)
Genome evolution
The bowfin genome contains an intact ParaHox gene cluster, similar to the bichir and to most other vertebrates. This is in contrast, however, with teleost fish, which have a fragmented ParaHox cluster, probably because of a whole genome duplication event in their lineage. The presence of an intact ParaHox gene cluster suggests that bowfin ancestors separated from other fish before the last common ancestor of all teleosts appeared. Bowfin are thus possibly a better model to study vertebrate genome organization than common teleost model organisms such as zebrafish.[48]
Feeding behavior

Bowfin are stalking, ambush predators that customarily move into the shallows at night to prey on fish, and aquatic invertebrates such as crawfish, mollusks, and aquatic insects.[49] Young bowfin feed mostly on small crustaceans, while adults are mostly piscivorous, but also known to be opportunistic.[50] Bowfin are remarkably agile, can move quickly through the water, and they have a voracious appetite.[5][13] Their undulating dorsal fin propels them silently through the water while stalking their prey. The attack is straightforward and swift with a movement that lasts approximately 0.075 seconds.[32] There were also some studies regarding the capacity of the bowfin to survive without food. In 1916, A female bowfin was starved for twenty months.[51] It was the longest period that any vertebrate had been without food, as far the writer was aware during the observation. Some independent studies focus on the bowfin's ability to use organic material as a source of food and studied the structure of the gill raker. They concluded that it did not benefit from the organic material in the water because the gill rakers were short with blunt processes and a short space between them. Even bacteria could enter and exit through the gill easily. Its structure alone indicated that the Amia don't use microorganisms as a source of food.
Distribution and habitat

Fossil deposits indicate amiiforms included freshwater and marine species that were once widely distributed in North America, South America, Eurasia and Africa.[52] Today, bowfin (Amia calva) are the only remaining species in the order Amiiformes; they are demersal freshwater piscivores, and their range is restricted to freshwater environments in North America, including much of the eastern United States and adjacent southern Canada from the St. Lawrence River and Lake Champlain drainage of southern Ontario and Quebec westward around the Great Lakes in southern Ontario into Minnesota.[53][54] Historically, their distribution in North America included the drainage basins of the Mississippi River from Quebec to northern Minnesota, the St. Lawrence-Great Lakes, including Georgian Bay, Lake Nipissing and Simcoe, Ontario, south to the Gulf of Mexico; Atlantic and Gulf Coastal Plain from the Susquehanna River drainage in southeastern Pennsylvania to the Colorado River in Texas.[4][53][55]
Stocking
Research from the late 1800s to the 1980s suggests a trend of intentional stockings of non-indigenous fish into ponds, lakes and rivers in the United States. At that time, little was known about environmental impacts, or long-term effects of new species establishment and spread as a result of "fish rescue and transfer" efforts, or the importance of nongame fish to the ecological balance of aquatic ecosystems.[56] Introductions of bowfin to areas they were considered a non-indigenous species included various lakes, rivers and drainages in Connecticut, Illinois, Iowa, Kansas, Kentucky Maryland, Massachusetts, Missouri, New Jersey, New York, North Carolina, Pennsylvania, Virginia, West Virginia, and Wisconsin.[53] Many of the introductions were intentional stockings by various resource management; however, there is no way to positively determine distribution resulting from flood transfers, or other inadvertent migrations. Bowfin are typically piscivorous, but as an introduced species are capable of being voracious predators that pose a threat to native fish and their prey.[53][57]
Preferred habitat
Bowfin prefer vegetated sloughs, lowland rivers and lakes, swamps, backwater areas, and are occasionally found in brackish water. They are well camouflaged, and not easy to spot in slow water with abundant vegetation. They often seek shelter under roots, and submerged logs.[50][58][59] Oxygen-poor environments can be tolerated because of their ability to breathe air.[50]
Life cycle
Bowfin spawn in the spring or early summer, typically between April and June, more commonly at night[32][50] in abundantly vegetated, clear shallow water in weed beds over sand bars, and also under stumps, logs, and bushes.[60] Optimum temperatures for nesting and spawning range between 16–19 °C (61–66 °F).[9] The males construct circular nests in fibrous root mats, clearing away leaves and stems. Depending on the density of surrounding vegetation there may be a tunnel-like entrance at one side.[60] The diameter of the nests commonly range between 39–91 cm (15–36 in),[9] at a water depth of 61–92 cm (24–36 in).[32]
During spawning season, the fins and underside of male bowfin often change in color to a bright lime green.[61] The courtship/spawning sequence lasts one to three hours, and can repeat up to five times.[32] Courtship begins when a female approaches the nest. The ritual consists of intermittent nose bites, nudges, and chasing behavior by the male until the female becomes receptive,[9] at which time the pair lie side by side in the nest. She deposits her eggs while he shakes his fins in a vibratory movement, and releases his milt for fertilization to occur.[9] A male often has eggs from more than one female in his nest, and a single female often spawns in several nests.[12][62]
Females vacate the nest after spawning,[32] leaving the male behind to protect the eggs during the eight to ten days of incubation.[13][60][63] A nest may contain 2,000 to 5,000 eggs, possibly more.[58] Fecundity is usually related to size of the fish, so it isn't unusual for the roe of a large gravid female to contain over 55,000 eggs.[32][50] Bowfin eggs are adhesive, and will attach to aquatic vegetation, roots, gravel, and sand.[32] After hatching, larval bowfin do not swim actively in search of food. During the seven to nine days required for yolk-sac absorption, they attach to vegetation by means of an adhesive organ on their snout, and remain protected by the parent male bowfin.[58] Bowfin aggressively protect their spawn from the first day of incubation to a month or so after the eggs have hatched.[58] When the fry are able to swim and forage on their own, they will form a school and leave the nest accompanied by the parent male bowfin who slowly circles them to prevent separation.[60]
Bowfin reach sexually maturity at two to three years of age.[50] They can live ten to twelve years in the wild,[50] and 30 years in captivity.[13][60] Females are longer-lived than males.[8]
Diseases

A common parasite of bowfin is the anchor worm (Lernaea). These small crustaceans infest the skin and bases of fins, with consequences ranging from slowed growth to death.[9] The mollusk Megalonaias gigantea lays eggs in the bowfin gills, that are then externally fertilized by sperm passing in the water flow. The small glochidia larvae then hatch and develop in the gill tubes.[9]
Bowfin with liver cancer and with fatal leukemia have been reported.[7]
Utilization
_(4015394787).jpg.webp)
As a sport fish, bowfin are not considered desirable to many anglers. They were once considered a nuisance fish by anglers and early biologists who believed the bowfin's predatory nature was harmful to sport fish populations. As a result, efforts were taken to reduce their numbers.[64] Research has since proven otherwise, and that knowledge together with a better understanding of maintaining overall balance of ecosystems, regulations were introduced to help protect and maintain viable populations of bowfin.[64] Bowfin are strong fighters, a prized trait in game fish. However, they do have a jaw full of sharp teeth which requires careful handling. The current tackle record is 21.5 lb (9.8 kg)[5][55][65]
Bowfin were once considered to have little commercial value because of its poor-tasting meat which has been referred to as "soft, bland-tasting and of poor texture".[5][13] However, it is considered quite palatable if cleaned properly and smoked, or prepared fried, blackened, used in courtbouillion, or in fishballs or fishcakes.[5][55][66] Over the years, global efforts have imposed strict regulations on the international trade of caviar, particularly on the harvest of sturgeons from the Caspian Sea where the highly prized caviar from the beluga sturgeon originates. The bans imposed on Caspian sturgeons have created lucrative markets for affordable substitutes in the United States including paddlefish, bowfin, and various species of sturgeon.[67] In Louisiana, bowfin are harvested in the wild, and cultured commercially in hatcheries for their meat and roe. The roe is processed into caviar, and sold as "Cajun caviar", or marketed under the trade name "Choupiquet Royale".[8][32][68]
Accumulation of toxic substances
In some areas of the United States where aquatic environments have tested positive for elevated levels of toxins, such as mercury, arsenic, chromium, and copper, there are posted signs with warnings about the consumption of fish caught in those areas.[69] Concentration of mercury biomagnifies as it passes up the food chain from organisms on lower trophic levels to apex predators. It bioaccumulates in the tissues of larger, long-lived predatory fish. When compared to smaller, short-lived fish, bowfin tend to concentrate mercury at higher levels thereby making them less safe for human consumption.[8][70]
References
- NatureServe (2015). "Amia calva". IUCN Red List of Threatened Species. 2015. Retrieved February 25, 2016.
- Froese, R.; Pauly, D. (2017). "Amiidae". FishBase version (02/2017). Retrieved 18 May 2017.
- "Amiidae" (PDF). Deeplyfish- fishes of the world. Retrieved 18 May 2017.
- Wisconsin DNR. "Bowfin Family-Amiidae" (PDF). University of Wisconsin. p. 254. Retrieved June 8, 2014.
- Kenneth Stewart; Douglas Watkinson (3 May 2004). Freshwater Fish of Manitoba. Univ. of Manitoba Press. p. 51. ISBN 978-0-88755-374-5.
- Froese, Rainer, and Daniel Pauly, eds. (2009). "Amiidae" in FishBase. January 2009 version.
- Jay Stauffer (1 December 2007). Fish of West Virginia. Academy of Natural Sciences. p. 40. ISBN 978-1-4223-1783-9.
- Johnathan G. Davis. "Reproductive Biology, Life History and Population Structure of a Bowfin Amia calva Population in Southeastern Louisiana, Fall 2003" (PDF). Nicholls State University. Archived from the original (PDF) on August 18, 2016. Retrieved June 7, 2014.
- University of Florida. "Bowfin". Ichthyology at the Florida Museum of Natural History. Florida Museum of Natural History. Retrieved June 11, 2014.
- Jeff Hansbarger (2006). "Bowfin" (PDF). Wildlife Diversity Notebook. West Virginia DNR. Retrieved September 25, 2014.
- Berra, Tim (September 15, 2008). Freshwater Fish Distribution. Families and Maps. University of Chicago Press. p. 52. ISBN 9780226044439.
- "Amia calva". Animal Diversity Web. University of Michigan. Retrieved September 25, 2014.
- Ken Schultz (15 December 2010). Ken Schultz's Field Guide to Freshwater Fish. John Wiley & Sons. p. 64. ISBN 978-1-118-03987-8.
- "Bowfin (Almia calva)" (PDF). Indiana Department of Natural Resources. Retrieved June 13, 2014.
- V., Kardong, Kenneth (2012-01-01). Vertebrates : comparative anatomy, function, evolution. McGraw-Hill. ISBN 9780073524238. OCLC 732361696.
- Jared Handley & Jesse Fielder (2004). "The Skeletal System of the Bowfin (Amia calva)". Murray State University.
- Nelson, Joseph S. (2006). Fish of the World. John Wiley & Sons, Inc. ISBN 978-0-471-25031-9.
- "Actinopterygians: What Are They?" (PDF). Biology of Fish-Fish/Biol 311. University of Washington. Retrieved August 8, 2014.
- Gene Helfman; Bruce B. Collette; Douglas E. Facey; Brian W. Bowen (3 April 2009). The Diversity of Fish: Biology, Evolution, and Ecology. John Wiley & Sons. p. 37. ISBN 978-1-4443-1190-7.
- "Northern Snakehead Fish". New York Department of Environmental Conservation. Retrieved July 22, 2014.
- "Bowfin and Snakeheads: Distinguishing Features". Brochure. Texas Parks & Wildlife Department. Retrieved August 4, 2014.
- Deborah Zabarenko (May 30, 2013). "Northern Snakehead Fish, Invasive Species, May Not Be As Bad As Originally Thought". Huffington Post Green. Reuters. Retrieved August 4, 2014.
- DNR (June 27, 2012). "Bowfin mistaken as snakeheads". Alerts and Notifications, event calendar press release. Indiana Department of Natural Resources. Retrieved June 14, 2014.
- Bureau of Fisheries Management. "Snakehead, Bowfin, or Burbot — Know the difference" (PDF). Wisconsin Anglers Want To Know brochures. Wisconsin Dept. of Natural Resources. Retrieved June 14, 2014.
- Kardong, Kenneth V (2015). Vertebrates: Comparative Anatomy, Function, Evolution. New York: McGraw-Hill Education.
- "Swimbladder" (PDF). Biology of Fish-Fish/Biol 311. University of Washington. Retrieved August 2, 2014.
- Hedrick MS, Jones DR (January 1999). "Control of gill ventilation and air-breathing in the bowfin amia calva". J. Exp. Biol. 202 (1): 87–94. PMID 9841898.
- D.C. Jackson, C.G. Farmer (March 5, 1998). "Air-Breathing During Activity in the Fish Lepisosteus Oculatus and Amia Calva" (PDF). The Journal of Experimental Biology (201): 943–948. Retrieved 8 June 2014.
- R.G. Boutilier (1990). "Control and Co-Ordination of Gas Exchange in Bimodal Breathers". Vertebrate Gas Exchange. Vertebrate Gas Exchange Advances in Comparative and Environmental Physiology, Volume 6, Chapter 9. Advances in Comparative and Environmental Physiology. 6. pp. 279–345. doi:10.1007/978-3-642-75380-0_9. ISBN 978-3-642-75382-4.
- David J. McKenzie; John F. Steffensen; Edwin W. Taylor & Augusto S. Abe (December 19, 2011). "The contribution of air breathing to aerobic scope and exercise performance in the banded knifefish Gymnotus carapo L". Journal of Experimental Biology. 215 (8): 1323–1330, Introduction. doi:10.1242/jeb.064543. PMID 22442370.
- Water Quality Assessment Division (2005). "Canon Envirothon Water Quality Study" (PDF). Louisiana Department of Environmental Quality. p. 18. Archived (PDF) from the original on April 13, 2013. Retrieved June 13, 2014.
- Stephen T. Ross (2001). The Inland Fish of Mississippi. Univ. Press of Mississippi. p. 94. ISBN 978-1-57806-246-1.
- Wilfred T. Neill (1950). Copeia, 1950, An estivating bowfin. American Society of Ichthyologists and Herpetologists. p. 240. ISBN 9780521739672.
- William F. Loftus; James A. Kushlan (1987). "Freshwater fish of southern Florida". Florida State Museum Biological Sciences. p. 183, Volume 31, No. 4.
- Wolfgang J. Plunk; Peter B. Bayley; Richard E. Sparks (1989). The Flood Pulse Concept in River-Floodplain Systems (PDF) (Report). University of Florida. pp. 117, 8–pdf.
- McKenzie, D. J.; Randall, D. J. (1990). "Does Amia calva aestivate?". Fish Physiology and Biochemistry. 8 (2): 147–58. doi:10.1007/BF00004442. PMID 24221948. S2CID 20401237.
- Gene Helfman; Bruce B. Collette; Douglas E. Facey; Brian W. Bowen (2009). The Diversity of Fish: Biology, Evolution, and Ecology. Wiley-Blackwell. p. 257, Chapter 13.
- Melvin L. Warren Jr.; Brooks M. Burr (July 2014). Freshwater Fish of North America. Volume 1: Petromyzontidae to Catostomidae. JHU Press. pp. 288–289. ISBN 9781421412016.
- Arratia, Gloria (2001). "The Sister-Group of Teleostei: Consensus And Disagreements". Journal of Vertebrate Paleontology. 21 (4): 767–773. doi:10.1671/0272-4634(2001)021[0767:TSGOTC]2.0.CO;2.
- Guang-Hui Xu; Li-Jun Zhao; Michael I. Coates (May 2014). "The oldest ionoscopiform from China sheds new light on the early evolution of halecomorph fish". Biology Letters. 10 (5): 20140204. doi:10.1098/rsbl.2014.0204. PMC 4046378. PMID 24872460.
- Imogen A. Hurley; Rachel Lockridge Mueller; Katherine A. Dunn; Eric J. Schmidt; Matt Friedman; Robert K. Ho; Victoria E. Prince; Ziheng Yang; Mark G. Thomas & Michael I. Coates (February 2007). "A New Time-Scale for Ray-Finned Fish Evolution". Proceedings of the Royal Society B: Biological Sciences. 274 (1609): 489–498. doi:10.1098/rspb.2006.3749. JSTOR 25223804. PMC 1766393. PMID 17476768.
- Broughton, Richard E. (2013). "Multi-locus phylogenetic analysis reveals the pattern and tempo of bony fish evolution". PLOS Currents. 5. doi:10.1371/currents.tol.2ca8041495ffafd0c92756e75247483e. PMC 3682800. PMID 23788273.
- Faircloth, Brant C. (2013). "A Phylogenomic Perspective on the Radiation of Ray-Finned Fish Based upon Targeted Sequencing of Ultraconserved Elements (UCEs)". PLOS ONE. 8 (6): e65923. Bibcode:2013PLoSO...865923F. doi:10.1371/journal.pone.0065923. PMC 3688804. PMID 23824177.
- "SUBCLASS ACTINOPTERYGII: RELICT SPECIES OF RAY-FINNED FISH & ORIGIN OF TELEOSTS". University of Edinburgh. January 2007. Retrieved September 29, 2014.
- Kanae Kikugawa; Kazutaka Katoh; Shigehiro Kuraku; Hiroshi Sakurai; Osamu Ishida; Naoyuki Iwabe; Takashi Miyata (March 11, 2004). "Basal jawed vertebrate phylogeny inferred from multiple nuclear DNA-coded genes". Research Article — Open Access. 2 (1): 3. doi:10.1186/1741-7007-2-3. PMC 387836. PMID 15070407.
- Thom Holmes (June 28, 2008). The First Vertebrate. Chelsea House Publishers. p. 144. ISBN 9780816059584.
- Haaramo, Mikko (2007). "Amiiformes – bowfin and relatives". Mikko's Phylogeny Archive. Retrieved 3 July 2017.
- John F. Mulley; Chi-hua Chiu; Peter W. H. Holland (2006). "Breakup of a homeobox cluster after genome duplication in teleosts". Proceedings of the National Academy of Sciences USA. 103 (27): 10369–10372. Bibcode:2006PNAS..10310369M. doi:10.1073/pnas.0600341103. PMC 1502464. PMID 16801555.
- Indiana Department of Fish & Wildlfife. "Bowfin (Amia calva)" (PDF). Indiana Department of Natural Resources. Retrieved June 13, 2014.
- Freshwater Fish of South Carolina. Univ of South Carolina Press. 2009. p. 80. ISBN 978-1-57003-680-4.
- Smallwood, W. M. (1916-01-01). "Twenty Months of Starvation in Amia Calva". Biological Bulletin. 31 (6): 453–464. doi:10.2307/1536322. JSTOR 1536322.
- Paul J.B. Hart; John D. Reynolds (2002). Handbook of Fish Biology and Fisheries. Wiley. p. 27. ISBN 978-0-632-05412-1.
- Fuller, Pam (April 11, 2006). "Amia calva". USGS Nonindigenous Aquatic Species Database. US Geological Survey. Retrieved August 9, 2014.
- "Amia calva (Bowfin)". IUCN Red List of Threatened Species. Retrieved September 29, 2014.
- John Acorn (7 February 2007). Deep Alberta: Fossil Facts and Dinosaur Digs. University of Alberta. p. 10. ISBN 978-0-88864-481-7.
- "Early Limnological and Fishery Research" (PDF). Wisconsin Fish and Fishery Management. University of Wisconsin. p. 29. Retrieved August 9, 2014.
- J. Richard Arthur; Rohana P. Subasinghe. "Potential Adverse Socio-Economic And Biological Impacts Of Aquatic Animal Pathogens Due To Hatchery-Based Enhancement Of Inland Open-Water Systems, And Possibilities For Their Minimisation". Primary aquatic animal health care in rural, small-scale, aquaculture. Inland Water Resources and Aquaculture Service. Retrieved August 9, 2014.
- Rudolph John Miller; Henry W. Robison (2004). Fish of Oklahoma. University of Oklahoma Press. p. 58. ISBN 978-0-8061-3610-3.
- Joshua Laerm; B. J. Freeman (January 2008). Fish of the Okefenokee Swamp. University of Georgia Press. p. 37. ISBN 978-0-8203-3135-5.
- Thomas, Chad; Bonner, Timothy; Whiteside, Timothy H. (June 18, 2007). Freshwater Fish of Texas. Texas A&M University Press. p. 23. ISBN 9781585445707. Retrieved July 1, 2017.
- Randy Jackson. "The Bowfin: New York's Disrespected Living Fossil". New York Department of Environmental Conservation. Retrieved June 11, 2014.
- Animals, jrank.org (1979). "Bowfins: Amiiformes – Physical Characteristics, Geographic Range, Habitat, Diet, Behavior and Reproduction, Bowfins and People, Conservation Status". Reference for the animal kingdom. Net Industries. Retrieved June 11, 2014.
- Berra, Tim M. (2001). Freshwater Fish Distribution. San Diego: Academic Press. ISBN 0-12-093156-7
- Randy Jackson. "The Bowfin, New York's Disrespected Living Fossil". New York Department of Environmental Conservation. Retrieved June 4, 2014.
- IGFA. "Bowfin". International Game Fish Association. Archived from the original on October 12, 2018. Retrieved June 4, 2014.
- David A. Bourgeois (April 5, 2009). "Choupique may be a trash fish for some, treasure to others". Daily Comet.
- Susan Saulny (June 9, 2012). "A Roe, By Any Other Name". New York Times.
- Christopher Scharpf (December 30, 2013). "Bowfin: North America's Freshwater Thug". USF&WS.
- EPA. "Fish Consumption Advisories". Environmental Protection Agency. Retrieved June 6, 2014.
- NCDPHS. "What fish are safe to eat?" (PDF). North Carolina Division of Public Health. Archived from the original (PDF) on September 20, 2013. Retrieved July 1, 2017.
Further reading
- McCormick, Catherine A. (1981). "Central Projects of the lateral line and eight nerves in the bowfin,Amia Calva" (PDF). The Journal of Comparative Neurology. 197 (1): 1–15. doi:10.1002/cne.901970102. hdl:2027.42/50011. PMID 6164698. S2CID 8836961.
- Conlon, J.M.; Youson, J.H.; Whittaker, J. (1991). "Structure and receptor-binding activity of insulin from a holostean fish, the bowfin:Amia Calva". Biochem. J. 276 (Pt 1): 261–264. doi:10.1042/bj2760261. PMC 1151174. PMID 2039477.
- Nguyen, T. M.; Mommsen, T. P.; Mims, S. M.; Conlon, J. M. (1994). "Characterization of insulins and proglucagon-derived peptides from a phylogenetically ancient fish, the paddlefish: Polyodon spathula". Biochem. J. 300 (2): 339–345. doi:10.1042/bj3000339. PMC 1138167. PMID 8002937.
- Conlon, J. M.; Youson, J. H.; Mommsen, T. P. (1993). "Structure and biological activity of glucagon and glucagon-like peptide from a primitive bony fish, the bowfin: Amia calva.". Biochem. J. 295 (3): 857–861. doi:10.1042/bj2950857. PMC 1134640. PMID 8240302.
- Sepkoski, Jack (2002). "A compendium of fossil marine animal genera". Bulletins of American Paleontology. 364: 560. Archived from the original on 2009-02-20. Retrieved 2011-05-17.
External links
| Wikispecies has information related to Amia calva. |
| Wikimedia Commons has media related to Amia calva. |
