Actinopterygii
Actinopterygii (/ˌæktɪnɒptəˈrɪdʒiaɪ/) (New Latin, actino- ("having rays") + Ancient Greek πτέρυξ (ptérux, "wing, fins")), members of which are known as ray-finned fishes, is a clade (traditionally class or subclass) of the bony fishes.[1]
| Ray-finned fish | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Superclass: | Osteichthyes |
| Class: | Actinopterygii Klein, 1885 |
| Subclasses | |
The ray-finned fishes are so-called because their fins are webs of skin supported by bony or horny spines ("rays"), as opposed to the fleshy, lobed fins that characterize the class Sarcopterygii (lobe-finned fish). These actinopterygian fin rays attach directly to the proximal or basal skeletal elements, the radials, which represent the link or connection between these fins and the internal skeleton (e.g., pelvic and pectoral girdles).
Numerically, actinopterygians are the dominant class of vertebrates, comprising nearly 99% of the over 30,000 species of fish.[2] They are ubiquitous throughout freshwater and marine environments from the deep sea to the highest mountain streams. Extant species can range in size from Paedocypris, at 8 mm (0.3 in), to the massive ocean sunfish, at 2,300 kg (5,070 lb), and the long-bodied oarfish, at 11 m (36 ft). The vast majority of Actinopterygii (~95%) are teleosts.
Characteristics
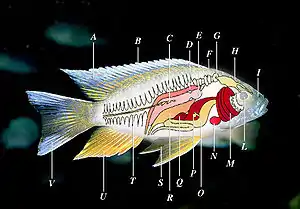
Ray-finned fishes occur in many variant forms. The main features of a typical ray-finned fish are shown in the adjacent diagram. The swim bladder is the more derived structure.[3]
Ray-finned fishes have many different types of scales; but all teleosts, the most advanced actinopterygians, have leptoid scales. The outer part of these scales fan out with bony ridges while the inner part is crossed with fibrous connective tissue. Leptoid scales are thinner and more transparent than other types of scales, and lack the hardened enamel or dentine-like layers found in the scales of many other fish. Unlike ganoid scales, which are found in non-teleost actinopterygians, new scales are added in concentric layers as the fish grows.
Ray-finned and lobe-finned fishes, including tetrapods, possessed lungs used for aerial respiration. Only bichirs retain ventrally budding lungs.[3]
Fin arrangements
Ray-finned fish are very varied in size and shape, and in the number of their ray-fins and the manner in which they arrange them.
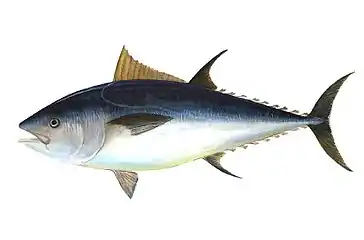 Tuna are streamlined for straight line speed with a deeply forked tail
Tuna are streamlined for straight line speed with a deeply forked tail The swordfish is even faster and more streamlined than the tuna
The swordfish is even faster and more streamlined than the tuna



 Fangtooth are indifferent swimmers who try to ambush their prey
Fangtooth are indifferent swimmers who try to ambush their prey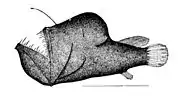 The first spine of the dorsal fin of anglerfish is modified like a fishing rod with a lure
The first spine of the dorsal fin of anglerfish is modified like a fishing rod with a lure
 King of herrings
King of herrings European conger are ray-finned fish
European conger are ray-finned fish.png.webp)


Reproduction

In nearly all ray-finned fish, the sexes are separate, and in most species the females spawn eggs that are fertilized externally, typically with the male inseminating the eggs after they are laid. Development then proceeds with a free-swimming larval stage.[4] However other patterns of ontogeny exist, with one of the commonest being sequential hermaphroditism. In most cases this involves protogyny, fish starting life as females and converting to males at some stage, triggered by some internal or external factor. Protandry, where a fish converts from male to female, is much less common than protogyny.[5] Most families use external rather than internal fertilization.[6] Of the oviparous teleosts, most (79%) do not provide parental care.[7] Viviparity, ovoviviparity, or some form of parental care for eggs, whether by the male, the female, or both parents is seen in a significant fraction (21%) of the 422 teleost families; no care is likely the ancestral condition.[7] Viviparity is relatively rare and is found in about 6% of teleost species; male care is far more common than female care.[7][8] Male territoriality "preadapts" a species for evolving male parental care.[9][10]
There are a few examples of fish that self-fertilise. The mangrove rivulus is an amphibious, simultaneous hermaphrodite, producing both eggs and spawn and having internal fertilisation. This mode of reproduction may be related to the fish's habit of spending long periods out of water in the mangrove forests it inhabits. Males are occasionally produced at temperatures below 19 °C (66 °F) and can fertilise eggs that are then spawned by the female. This maintains genetic variability in a species that is otherwise highly inbred.[11]
Fossil record
The earliest known fossil actinopterygian is Andreolepis hedei, dating back 420 million years (Late Silurian). Remains have been found in Russia, Sweden, and Estonia.[12]
Classification
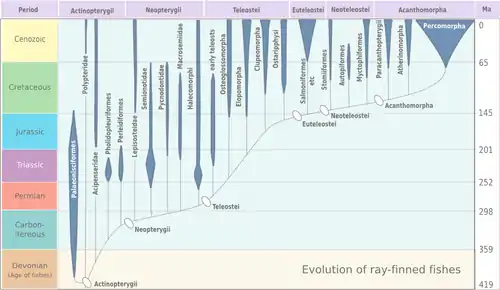
Actinopterygia is divided into the subclasses Chondrostei and Neopterygii. The Neopterygii, in turn, is divided into the infraclasses Holostei and Teleostei. During the Mesozoic and Cenozoic the teleosts in particular diversified widely, and as a result, 96% of all known fish species are teleosts. The cladogram shows the major groups of actinopterygians and their relationship to the terrestrial vertebrates (tetrapods) that evolved from a related group of fish.[13][14][15] Approximate dates are from Near et al., 2012.[13]
| Osteichthyes |
| ||||||||||||||||||||||||||||||||||||||||||||||||
The polypterids (bichirs and reedfish) are the sister lineage of all other actinopterygians, the Acipenseriformes (sturgeons and paddlefishes) are the sister lineage of Neopterygii, and Holostei (bowfin and gars) are the sister lineage of teleosts. The Elopomorpha (eels and tarpons) appear to be the most basal teleosts.[13]
| Chondrostei | Chondrostei (cartilage bone) is a subclass of primarily cartilaginous fish showing some ossification. Earlier definitions of Chondrostei are now known to be paraphyletic, meaning that this subclass does not contain all the descendants of their common ancestor. There were 52 species divided among two orders, the Acipenseriformes (sturgeons and paddlefishes) and the Polypteriformes (reedfishes and bichirs). Reedfish and birchirs are now separated from the Chondrostei into their own sister lineage, the Cladistia. It is thought that the chondrosteans evolved from bony fish but lost the bony hardening of their cartilaginous skeletons, resulting in a lightening of the frame. Elderly chondrosteans show beginnings of ossification of the skeleton, suggesting that this process is delayed rather than lost in these fish.[16] This group had once been classified with the sharks: the similarities are obvious, as not only do the chondrosteans mostly lack bone, but the structure of the jaw is more akin to that of sharks than other bony fish, and both lack scales (excluding the Polypteriforms). Additional shared features include spiracles and, in sturgeons, a heterocercal tail (the vertebrae extend into the larger lobe of the caudal fin). However the fossil record suggests that these fish have more in common with the Teleostei than their external appearance might suggest.[16] | |
|---|---|---|
| Neopterygii | Neopterygii (new fins) is a subclass of ray-finned fish that appeared somewhere in the Late Permian. There were only few changes during its evolution from the earlier actinopterygians. Neopterygians are a very successful group of fishes because they can move more rapidly than their ancestors. Their scales and skeletons began to lighten during their evolution, and their jaws became more powerful and efficient. While electroreception and the ampullae of Lorenzini is present in all other groups of fish, with the exception of hagfish, neopterygians have lost this sense, though it later re-evolved within Gymnotiformes and catfishes, who possess nonhomologous teleost ampullae.[17] |




The listing below follows Phylogenetic Classification of Bony Fishes[14][18] with notes when this differs from Nelson,[19] ITIS[20] and FishBase[21] and extinct groups from Van der Laan 2016.[22]
- Order †?Asarotiformes Schaeffer 1968
- Order †?Discordichthyiformes Minikh 1998
- Order †?Paphosisciformes Grogan & Lund 2015
- Order †?Scanilepiformes Selezneya 1985
- Order †Cheirolepidiformes Kazantseva-Selezneva 1977
- Order †Paramblypteriformes Heyler 1969
- Order †Rhadinichthyiformes
- Order †Palaeonisciformes Hay 1902
- Order †Tarrasiiformes sensu Lund & Poplin 2002
- Order †Ptycholepiformes Andrews et al. 1967
- Order †Redfieldiiformes Berg 1940
- Order †Haplolepidiformes Westoll 1944
- Order †Aeduelliformes Heyler 1969
- Order †Platysomiformes Aldinger 1937
- Order †Dorypteriformes Cope 1871
- Order †Eurynotiformes Sallan & Coates 2013
- Subclass Cladistii Pander 1860
- Order †Guildayichthyiformes Lund 2000
- Order Polypteriformes Bleeker 1859 (bichirs and reedfishes)[23]
- Clade Actinopteri Cope 1972 s.s.
- Order †Elonichthyiformes Kazantseva-Selezneva 1977
- Order †Phanerorhynchiformes
- Order †Saurichthyiformes Berg 1937
- Subclass Chondrostei
- Order †Birgeriiformes Jin 2001
- Order †Chondrosteiformes
- Order Acipenseriformes Berg 1940 (sturgeons and paddlefishes)
- Subclass Neopterygii Regan 1923 sensu Xu & Wu 2012
- Order †Pholidopleuriformes Berg 1937
- Order †Peltopleuriformes Lehman 1966
- Order †Perleidiformes Berg 1937
- Order †Luganoiiformes Lehman 1958
- Order †Pycnodontiformes Berg 1937
- Infraclass Holostei Muller 1844
- Division Halecomorpha Cope 1872 sensu Grande & Bemis 1998
- Order †Parasemionotiformes Lehman 1966
- Order †Ionoscopiformes Grande & Bemis 1998
- Order Amiiformes Huxley 1861 sensu Grande & Bemis 1998 (bowfins)
- Division Ginglymodi Cope 1871
- Order †Dapediiformes Thies & Waschkewitz 2015
- Order †Semionotiformes Arambourg & Bertin 1958
- Order Lepisosteiformes Hay 1929 (gars)
- Division Halecomorpha Cope 1872 sensu Grande & Bemis 1998
- Clade Teleosteomorpha Arratia 2000 sensu Arratia 2013
- Order †Prohaleciteiformes Arratia 2017
- Division Aspidorhynchei Nelson, Grand & Wilson 2016
- Order †Aspidorhynchiformes Bleeker 1859
- Order †Pachycormiformes Berg 1937
- Infraclass Teleostei Müller 1844 sensu Arratia 2013
- Order †?Araripichthyiformes
- Order †?Ligulelliiformes Taverne 2011
- Order †?Tselfatiiformes Nelson 1994
- Order †Pholidophoriformes Berg 1940
- Order †Dorsetichthyiformes Nelson, Grand & Wilson 2016
- Order †Leptolepidiformes
- Order †Crossognathiformes Taverne 1989
- Order †Ichthyodectiformes Bardeck & Sprinkle 1969
- Teleocephala de Pinna 1996 s.s.
- Megacohort Elopocephalai Patterson 1977 sensu Arratia 1999 (Elopomorpha Greenwood et al. 1966)
- Order Elopiformes Gosline 1960 (ladyfishes and tarpon)
- Order Albuliformes Greenwood et al. 1966 sensu Forey et al. 1996 (bonefishes)
- Order Notacanthiformes Goodrich 1909 (halosaurs and spiny eels)
- Order Anguilliformes Jarocki 1822 sensu Goodrich 1909 (true eels)
- Megacohort Osteoglossocephalai sensu Arratia 1999
- Supercohort Osteoglossocephala sensu Arratia 1999 (Osteoglossomorpha Greenwood et al. 1966)
- Order †Lycopteriformes Chang & Chou 1977
- Order Hiodontiformes McAllister 1968 sensu Taverne 1979 (mooneye and goldeye)
- Order Osteoglossiformes Regan 1909 sensu Zhang 2004 (bony-tongued fishes)
- Supercohort Clupeocephala Patterson & Rosen 1977 sensu Arratia 2010
- Cohort Otomorpha Wiley & Johnson 2010 (Otocephala; Ostarioclupeomorpha)
- Subcohort Clupei Wiley & Johnson 2010 (Clupeomorpha Greenwood et al. 1966)
- Order †Ellimmichthyiformes Grande 1982
- Order Clupeiformes Bleeker 1859 (herrings and anchovies)
- Subcohort Alepocephali
- Order Alepocephaliformes Marshall 1962
- Subcohort Ostariophysi Sagemehl 1885
- Section Anotophysa (Rosen & Greenwood 1970) Sagemehl 1885
- Order †Sorbininardiformes Taverne 1999
- Order Gonorynchiformes Regan 1909 (milkfishes)
- Section Otophysa Garstang 1931
- Order Cypriniformes Bleeker 1859 sensu Goodrich 1909 (barbs, carp, danios, goldfishes, loaches, minnows, rasboras)
- Order Characiformes Goodrich 1909 (characins, pencilfishes, hatchetfishes, piranhas, tetras, dourado / golden (genus Salminus) and pacu)
- Order Gymnotiformes Berg 1940 (electric eels and knifefishes)
- Order Siluriformes Cuvier 1817 sensu Hay 1929 (catfishes)
- Section Anotophysa (Rosen & Greenwood 1970) Sagemehl 1885
- Subcohort Clupei Wiley & Johnson 2010 (Clupeomorpha Greenwood et al. 1966)
- Cohort Euteleosteomorpha (Greenwood et al. 1966) (Euteleostei Greenwood 1967 sensu Johnson & Patterson 1996)
- Subcohort Lepidogalaxii
- Lepidogalaxiiformes Betancur-Rodriguez et al. 2013 (salamanderfish)
- Subcohort Protacanthopterygii Greenwood et al. 1966 sensu Johnson & Patterson 1996
- Order Argentiniformes (barreleyes and slickheads) (formerly in Osmeriformes)
- Order Galaxiiformes
- Order Salmoniformes Bleeker 1859 sensu Nelson 1994 (salmon and trout)
- Order Esociformes Bleeker 1859 (pike)
- Subcohort Stomiati
- Order Osmeriformes (smelts)
- Order Stomiatiformes Regan 1909 (bristlemouths and marine hatchetfishes)
- Subcohort Neoteleostei Nelson 1969
- Infracohort Ateleopodia
- Order Ateleopodiformes (jellynose fish)
- Infracohort Eurypterygia Rosen 1973
- Section Aulopa [Cyclosquamata Rosen 1973]
- Order Aulopiformes Rosen 1973 (Bombay duck and lancetfishes)
- Section Ctenosquamata Rosen 1973
- Subsection Myctophata [Scopelomorpha]
- Order Myctophiformes Regan 1911 (lanternfishes)
- Subsection Acanthomorpha Betancur-Rodriguez et al. 2013
- Division Lampridacea Betancur-Rodriguez et al. 2013 [Lampridomorpha; Lampripterygii]
- Order Lampriformes Regan 1909 (oarfish, opah and ribbonfishes)
- Division Paracanthomorphacea sensu Grande et al. 2013 (Paracanthopterygii Greenwood 1937)
- Order Percopsiformes Berg 1937 (cavefishes and trout-perches)
- Order †Sphenocephaliformes Rosen & Patterson 1969
- Order Zeiformes Regan 1909 (dories)
- Order Stylephoriformes Miya et al. 2007
- Order Gadiformes Goodrich 1909 (cods)
- Division Polymixiacea Betancur-Rodriguez et al. 2013 (Polymyxiomorpha; Polymixiipterygii)
- Order †Pattersonichthyiformes Gaudant 1976
- Order †Ctenothrissiformes Berg 1937
- Order Polymixiiformes Lowe 1838 (beardfishes)
- Division Euacanthomorphacea Betancur-Rodriguez et al. 2013 (Euacanthomorpha sensu Johnson & Patterson 1993; Acanthopterygii Gouan 1770 sensu])
- Subdivision Berycimorphaceae Betancur-Rodriguez et al. 2013
- Order Beryciformes (fangtooths and pineconefishes) (incl. Stephanoberyciformes; Cetomimiformes)
- Subdivision Holocentrimorphaceae Betancur-Rodriguez et al. 2013
- Order Holocentriformes (Soldierfishes)
- Subdivision Percomorphaceae Betancur-Rodriguez et al. 2013 (Percomorpha sensu Miya et al. 2003; Acanthopteri)
- Series Ophidiimopharia Betancur-Rodriguez et al. 2013
- Order Ophidiiformes (pearlfishes)
- Series Batrachoidimopharia Betancur-Rodriguez et al. 2013
- Order Batrachoidiformes (toadfishes)
- Series Gobiomopharia Betancur-Rodriguez et al. 2013
- Order Kurtiformes(Nurseryfishes and cardinalfishes)
- Order Gobiiformes(Sleepers and gobies)
- Series Scombrimopharia Betancur-Rodriguez et al. 2013
- Order Syngnathiformes (seahorses, pipefishes, sea moths, cornetfishes and flying gurnards[24])
- Order Scombriformes (Tunas and (mackerels)
- Series Carangimopharia Betancur-Rodriguez et al. 2013
- Subseries Anabantaria Betancur-Rodriguez et al. 2014
- Order Synbranchiformes (swamp eels)
- Order Anabantiformes (Labyrinthici) (gouramies, snakeheads, )
- Subseries Carangaria Betancur-Rodriguez et al. 2014
- Carangaria incertae sedis
- Order Istiophoriformes Betancur-Rodriguez 2013 (Marlins, swordfishes, billfishes)
- Order Carangiformes (Jack mackerels, pompanos)
- Order Pleuronectiformes Bleeker 1859 (flatfishes)
- Subseries Ovalentaria Smith & Near 2012 (Stiassnyiformes sensu Li et al. 2009)
- Ovalentaria incertae sedis
- Order Cichliformes Betancur-Rodriguez et al. 2013 (Cichlids, Convict blenny, leaf fishes)
- Order Atheriniformes Rosen 1964 (silversides and rainbowfishes)
- Order Cyprinodontiformes Berg 1940 (livebearers, killifishes)
- Order Beloniformes Berg 1940 (flyingfishes and ricefishes)
- Order Mugiliformes Berg 1940 (mullets)
- Order Blenniiformes Springer 1993 (Blennies)
- Order Gobiesociformes Gill 1872 (Clingfishes)
- Subseries Anabantaria Betancur-Rodriguez et al. 2014
- Series Eupercaria Betancur-Rodriguez et al. 2014 (Percomorpharia Betancur-Rodriguez et al. 2013)
- Eupercaria incertae sedis
- Order Gerreiformes (Mojarras)
- Order Labriformes (Wrasses and Parrotfishes)
- Order Caproiformes (Boarfishes)
- Order Lophiiformes Garman 1899 (Anglerfishes)
- Order Tetraodontiformes Regan 1929 (Filefishes and pufferfish)
- Order Centrarchiformes Bleeker 1859 (Sunfishes and mandarin fishes)
- Order Gasterosteiformes (Sicklebacks and relatives)
- Order Scorpaeniformes (Lionfishes and relatives)
- Order Perciformes Bleeker 1859
- Series Ophidiimopharia Betancur-Rodriguez et al. 2013
- Subdivision Berycimorphaceae Betancur-Rodriguez et al. 2013
- Division Lampridacea Betancur-Rodriguez et al. 2013 [Lampridomorpha; Lampripterygii]
- Subsection Myctophata [Scopelomorpha]
- Section Aulopa [Cyclosquamata Rosen 1973]
- Infracohort Ateleopodia
- Subcohort Lepidogalaxii
- Cohort Otomorpha Wiley & Johnson 2010 (Otocephala; Ostarioclupeomorpha)
- Supercohort Osteoglossocephala sensu Arratia 1999 (Osteoglossomorpha Greenwood et al. 1966)
- Megacohort Elopocephalai Patterson 1977 sensu Arratia 1999 (Elopomorpha Greenwood et al. 1966)
References
- Kardong, Kenneth (2015). Vertebrates: Comparative Anatomy, Function, Evolution. New York: McGraw-Hill Education. pp. 99–100. ISBN 978-0-07-802302-6.
- (Davis, Brian 2010).
- Funk, Emily; Breen, Catriona; Sanketi, Bhargav; Kurpios, Natasza; McCune, Amy (2020). "Changing in Nkx2.1, Sox2, Bmp4, and Bmp16 expression underlying the lung-to-gas bladder evolutionary transition in ray-finned fishes". Evolution & Development. 22 (5): 384–402.
- Dorit, R.L.; Walker, W.F.; Barnes, R.D. (1991). Zoology. Saunders College Publishing. p. 819. ISBN 978-0-03-030504-7.
- Avise, J.C.; Mank, J.E. (2009). "Evolutionary perspectives on hermaphroditism in fishes". Sexual Development. 3 (2–3): 152–163. doi:10.1159/000223079. PMID 19684459.
- Pitcher, T (1993). The Behavior of Teleost Fishes. London: Chapman & Hall.
- Reynolds, John; Nicholas B. Goodwin; Robert P. Freckleton (19 March 2002). "Evolutionary Transitions in Parental Care and Live Bearing in Vertebrates". Philosophical Transactions of the Royal Society B: Biological Sciences. 357 (1419): 269–281. doi:10.1098/rstb.2001.0930. PMC 1692951. PMID 11958696.
- Clutton-Brock, T. H. (1991). The Evolution of Parental Care. Princeton, NJ: Princeton UP.
- Werren, John; Mart R. Gross; Richard Shine (1980). "Paternity and the evolution of male parentage". Journal of Theoretical Biology. 82 (4): 619–631. doi:10.1016/0022-5193(80)90182-4. PMID 7382520. Retrieved 15 September 2013.
- Baylis, Jeffrey (1981). "The Evolution of Parental Care in Fishes, with reference to Darwin's rule of male sexual selection". Environmental Biology of Fishes. 6 (2): 223–251. doi:10.1007/BF00002788.
- Wootton, Robert J.; Smith, Carl (2014). Reproductive Biology of Teleost Fishes. Wiley. ISBN 978-1-118-89139-1.
- "Fossilworks: Andreolepis". Archived from the original on 12 February 2010. Retrieved 14 May 2008.
- Thomas J. Near; et al. (2012). "Resolution of ray-finned fish phylogeny and timing of diversification". PNAS. 109 (34): 13698–13703. Bibcode:2012PNAS..10913698N. doi:10.1073/pnas.1206625109. PMC 3427055. PMID 22869754. Archived from the original on 11 April 2015.
- Betancur-R, Ricardo; et al. (2013). "The Tree of Life and a New Classification of Bony Fishes". PLOS Currents Tree of Life. 5 (Edition 1). doi:10.1371/currents.tol.53ba26640df0ccaee75bb165c8c26288. PMC 3644299. PMID 23653398. Archived from the original on 13 October 2013.
- Laurin, M.; Reisz, R.R. (1995). "A reevaluation of early amniote phylogeny". Zoological Journal of the Linnean Society. 113 (2): 165–223. doi:10.1111/j.1096-3642.1995.tb00932.x.
- "Chondrosteans: Sturgeon Relatives". paleos.com. Archived from the original on 25 December 2010.
- Theodore Holmes Bullock; Carl D. Hopkins; Arthur N. Popper (2005). Electroreception. Springer Science+Business Media, Incorporated. p. 229. ISBN 978-0-387-28275-6.
- Betancur-Rodriguez; et al. (2017). "Phylogenetic Classification of Bony Fishes Version 4". BMC Evolutionary Biology. 17 (1): 162. doi:10.1186/s12862-017-0958-3. PMC 5501477. PMID 28683774.
- Nelson, Joseph, S. (2016). Fishes of the World. John Wiley & Sons, Inc. ISBN 978-1-118-34233-6.
- "Actinopterygii". Integrated Taxonomic Information System. Retrieved 3 April 2006.
- R. Froese and D. Pauly, editors (February 2006). "FishBase". Archived from the original on 5 July 2018. Retrieved 8 January 2020.
- Van der Laan, Richard (2016). Family-group names of fossil fishes. doi:10.13140/RG.2.1.2130.1361.
- In Nelson, Polypteriformes is placed in its own subclass Cladistia.
- In Nelson and ITIS, Syngnathiformes is placed as the suborder Syngnathoidei of the order Gasterosteiformes.
External links
 Media related to Actinopterygii at Wikimedia Commons
Media related to Actinopterygii at Wikimedia Commons Data related to Actinopterygii at Wikispecies
Data related to Actinopterygii at Wikispecies