CYR61
Cysteine-rich angiogenic inducer 61 (CYR61) or CCN family member 1 (CCN1), is a matricellular protein that in humans is encoded by the CYR61 gene.[5]
CYR61 is a secreted, extracellular matrix (ECM)-associated signaling protein of the CCN family (CCN intercellular signaling protein).[6][7] CYR61 is capable of regulating a broad range of cellular activities, including cell adhesion, migration, proliferation, differentiation, apoptosis, and senescence through interaction with cell surface integrin receptors and heparan sulfate proteoglycans. During embryonic development, CYR61 is critical for cardiac septal morphogenesis, blood vessel formation in placenta, and vascular integrity. In adulthood CYR61 plays important roles in inflammation and tissue repair, and is associated with diseases related to chronic inflammation, including rheumatoid arthritis, atherosclerosis, diabetes-related nephropathy and retinopathy, and many different forms of cancers.
CCN protein family
CYR61 was first identified as a protein encoded by a serum-inducible gene in mouse fibroblasts.[6][8] Other highly conserved homologs were later identified to comprise the CCN protein family (CCN intercellular signaling protein).[9][10][11] The CCN acronym is derived from the first three members of the family identified, namely CYR61 (CCN1), CTGF (connective tissue growth factor, or CCN2), and NOV (nephroblastoma overexpressed, or CCN3). These proteins, together with WISP1 (CCN4), WISP2 (CCN5), and WISP3 (CCN6) comprise the six members of the family in vertebrates and have been renamed CCN1-6 in order of their discovery by international consensus.[12] CCN proteins function as matricellular proteins, which are extracellular matrix proteins that play regulatory roles, particularly in the context of wound repair.[13]
Gene structure and regulation
CYR61 is located at human chromosome 1p22.3, whereas the mouse Cyr61 gene is located at chromosome 3, 72.9cM.[14] The mouse CYR61 coding region spans ~3.2 Kb, containing 5 exons interspaced with 4 introns.[15] The first exon encodes 5’-UTR sequence and the first several amino acids in the secretory signal peptide. The remaining four exons each encode a distinct CCN1 domain. The 5th exon also contains the 3’-UTR sequences, which has 5 copies of AU-rich elements that confers a short mRNA half life, and a mir-155 target site.[16] The CYR61 promoter is a TATA box containing promoter, with binding sites for many transcription factors including AP1, ATF, E2F, HNF3b, NF1, NFκB, SP1, and SRF, and 2 poly(CA) stretches that may form Z-DNA structure. Transcriptional activation of CYR61 is exquisitely sensitive to a wide range of environmental perturbations, including stimulation by platelet-derived growth factor and basic fibroblast growth factor, transforming growth factor β1 (TGF-β1), growth hormone, the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA), cAMP, vitamin D3, estrogen and tamoxifen, angiotensin II, hypoxia, UV light, and mechanical stretch.[7][11]
Protein structure and function
Structural domains
Full-length CYR61 protein contains 381 amino acids with an N-terminal secretory signal peptide followed by four structurally distinct domains.[17] The four CYR61 domains are, from N- to C-termini, the insulin-like growth factor binding protein (IGFBP) domain, von Willebrand type C repeats (vWC) domain, thrombospondin type 1 repeat domain (TSR), and the C-terminal (CT) domain that contains a cysteine-knot motif. CCN1 has unusually high cysteine residue content (10% or 38 in total). The number and spacing of cysteine residues are completely conserved among CYR61 (CCN1), CTGF (CCN2), NOV (CCN3), and WISP-1 (CCN4), and are largely conserved with WISP-2 (CCN5), which lacks precisely the CT domain, and WISP3 (CCN6), which lacks 4 cysteines in the vWC domain. CYR61 is glycosylated, although the regulation and function of gylcosylation are unknown.
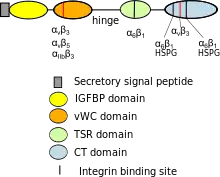
Integrin binding
CYR61 binds directly to various integrin receptors in a cell type-dependent manner, including integrin αvβ3 in endothelial cells,[18] α6β1 and heparan sulfate proteoglycans (HSPGs) in fibroblasts and smooth muscle cells[19][20] αIIbβ3 in activated platelets,[21] αMβ2 in monocytes and macrophages,[22][23] and αDβ2 in macrophage foam cells.[24] Where examined, syndecan-4 has been identified as the HSPG critical for CCN1 functions.[25][26] The CYR61 binding sites for some of these integrins have been mapped (Figure 1). Due to the cell type specificity of integrin expression, CYR61 acts through distinct integrins to mediate specific functions in different types of cells. For example, CYR61 induces angiogenic functions in endothelial cells through αvβ3,[27] and in fibroblasts promotes cellular senescence and enables TNFα to induce apoptosis through binding to α6β1-HSPGs.[26][28] However, CYR61 supports cell adhesion through all of the integrins identified above.
Cell signaling and function
As a cell adhesive substrate, CYR61 induces the activation of focal adhesion kinase, paxillin, RAC, and sustained activation of MAPK/ERK1-2.[29] In macrophages, CYR61 also activates the transcription factor NFκB and stimulates M1 polarization.[23] CYR61 activates Akt signaling in thymic epithelial cells, promoting their proliferation and thus thymic size growth.[30] CYR61 has potent angiogenic activity upon endothelial cells and induces neovascularization, first demonstrated in a corneal micropocket implant assay[31] and subsequently confirmed in a rabbit ischemic hindlimb model.[32] CYR61 also accelerates and promotes the chondrogenic differentiation of mouse limb bud mesenchymal cells,[33] and stimulates osteoblast differentiation but inhibits osteoclastogenesis.[34][35][36] Cyr61 is a strong inducer of reactive oxygen species accumulation in fibroblastic cells, and this activity underlies many CYR61-induced apoptosis and senescence.[26][28] CYR61 is able to support cell adhesion, stimulate cell migration, promote growth factor-induced cell proliferation and differentiation in some cell types, promote apoptosis in synergy with TNF family cytokines, and induce cellular senescence in fibroblasts.
Embryonic development
During embryo development in mice, Cyr61 is highly expressed in the cardiovascular, skeletal, and neuronal systems.[37][38] Cyr61 knockout mice are embryonic lethal due to defects in cardiac septal morphogenesis, deficient blood vessel formation in placenta, and compromised vascular integrity.[39][40] In Xenopus laevis, Cyr61 is required for normal gastrulation and modulation of Wnt signaling.[41]
Clinical relevance
CYR61 is highly expressed at sites of inflammation and wound repair, and is associated with diseases involving chronic inflammation and tissue injury.[7]
Wound healing and fibrosis
In skin wound healing, CYR61 is highly expressed in the granulation tissue by myofibroblasts, which proliferate and rapidly synthesize ECM to maintain tissue integrity and to promote regeneration of parenchymal cells.[42][43] However, excessive matrix deposition can lead to fibrosis, scarring, and loss of tissue function. In skin wounds, CYR61 accumulates in the granulation tissue as myofibroblasts proliferate, and eventually reaches a sufficiently high level to drive the myofibroblasts themselves into senescence, whereupon these cells cease to proliferate and express matrix-degrading enzymes.[28] Thus, CYR61 limits synthesis and deposition of ECM by myofibroblasts, reducing the risk of fibrosis during wound healing.[44] In addition to skin wound healing, CYR61 expression is elevated in remodeling cardiomyocytes after myocardial infarction,[45] in vascular injury,[20] and in the long bones during fracture repair.[46][47] Blockade of CYR61 by antibodies inhibits bone fracture healing in mice.[48] In the kidney, CYR61 is expressed in podocytes in normal adult and embryonic glomeruli, but expression is decreased in IgA nephropathy, diabetic nephropathy, and membranous nephropathy, particularly in diseased kidneys with severe mesangial expansion.[49]
Inflammation
CYR61 promotes the apoptotic functions of inflammatory cytokines such as TNFα, FasL, and TRAIL.[26][50][51] It also reprograms macrophages towards M1 polarization through αMβ2-mediated activation of NF-κB.[23] CYR61 is upregulated in patients with Crohn's disease and ulcerative colitis.[52] CYR61 supports the patrolling behavior of murine resident Ly6Clow monocytes along the endothelial in the steady state and is required for their accumulation under viral-mimicking vascular inflammation.[53]
Arthritis
CYR61 is highly expressed in collagen-induced arthritis in rodents, and inhibition of CCN1 expression correlates with suppression of inflammatory arthritis.[54] CYR61 is also found in articular cartilage from patients with osteoarthritis and appears to suppress ADAMTS4 (aggrecanase) activity, possibly leading to cartilage cell (chondrocyte) cloning.[55]
Vascular diseases
CYR61 is overexpressed in vascular smooth muscle cells of atherosclerotic lesions and in the neointima of restenosis after balloon angioplasty, both in rodent models and in humans.[20][22][56][57] Suppression of CYR61 expression results in reduced neointimal hyperplasia after balloon angioplasty, an effect that is reversed by delivery of CYR61 via gene transfer[58][59] In a mouse model of oxygen-induced retinopathy, expression of CYR61 in the vitreous humor produced significant beneficial effects in repairing damaged vasculature.[60]
Cancer
Angiogenesis is essential for the supply of oxygen and nutrients to nourish the growing tumor.[61] CYR61 is a powerful angiogenic inducer in vivo,[31][32] and it can also promote cancer cell proliferation, invasion, survival, epithelial–mesenchymal transition, and metastasis.[62][63][64][65] Accordingly, forced overexpression of CYR61 enhanced tumor growth in xenografts of breast cancer cells,[66] prostate cancer cells,[63] ovarian carcinoma cells,[67] and squamous carcinoma cells.[68] Clinically, CYR61 expression correlates with the tumor stage, tumor size, lymph node positivity, and poor prognosis in several cancers, including breast cancer,[66][69][70][71][72] prostate cancer,[73] glioma,[74] gastric adenocarcinoma,[75] and squamous cell carcinoma.[68]
However, CYR61 can also induce apoptosis and cellular senescence,[25][28][76] two well-established mechanisms of tumor suppression[77] Thus, whereas CYR61 can promote the proliferation of prostate cancer cells, it can also exacerbate apoptosis of these cells in the presence of the immune surveillance molecule TRAIL.[51][63][78] CYR61 has an inhibitory effect on some cancers, and suppresses tumor growth of non-small-cell lung cancer (NSCLC) cells,[79] endometrial adenocarcinoma cells,[80] and in melanoma cells.[81]
References
- GRCh38: Ensembl release 89: ENSG00000142871 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000028195 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Jay P, Berge-Lefranc JL, Marsollier C, Mejean C, Taviaux S, Berta P (May 1997). "The human growth factor-inducible immediate early gene, CYR61, maps to chromosome 1p". Oncogene. 14 (14): 1753–7. doi:10.1038/sj.onc.1200986. PMID 9135077.
- Lau LF (October 2011). "CCN1/CYR61: the very model of a modern matricellular protein". Cell. Mol. Life Sci. 68 (19): 3149–63. doi:10.1007/s00018-011-0778-3. PMC 3651699. PMID 21805345.
- Jun JI, Lau LF (December 2011). "Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets". Nature Reviews Drug Discovery. 10 (12): 945–63. doi:10.1038/nrd3599. PMC 3663145. PMID 22129992.
- Lau LF, Nathans D (1985). "Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells". EMBO J. 4 (12): 3145–3151. doi:10.1002/j.1460-2075.1985.tb04057.x. PMC 554634. PMID 3841511.
- Leask A, Abraham DJ (2006). "All in the CCN family: essential matricellular signaling modulators emerge from the bunker". J. Cell Sci. 119 (Pt 23): 4803–4810. doi:10.1242/jcs.03270. PMID 17130294.
- Holbourn KP, Acharya KR, Perbal B (2008). "The CCN family of proteins: structure-function relationships". Trends Biochem. Sci. 33 (10): 461–473. doi:10.1016/j.tibs.2008.07.006. PMC 2683937. PMID 18789696.
- Chen CC, Lau LF (2009). "Functions and Mechanisms of Action of CCN Matricellular Proteins". Int. J. Biochem. Cell Biol. 41 (4): 771–783. doi:10.1016/j.biocel.2008.07.025. PMC 2668982. PMID 18775791.
- Brigstock DR, Goldschmeding R, Katsube KI, Lam SC, Lau LF, Lyons K, Naus C, Perbal B, Riser B (2003). "Proposal for a unified CCN nomenclature". Mol. Pathol. 56 (2): 127–128. doi:10.1136/mp.56.2.127. PMC 1187305. PMID 12665631.
- Bornstein P, Sage EH (2002). "Matricellular proteins: extracellular modulators of cell function". Current Opinion in Cell Biology. 14 (5): 608–616. doi:10.1016/S0955-0674(02)00361-7. PMID 12231357.
- Jay P, Berge-Lefranc JL, Marsollier C, Mejean C, Taviaux S, Berta P (1997). "The human growth factor-inducible immediate early gene, CYR61, maps to chromosome 1p". Oncogene. 14 (14): 1753–1757. doi:10.1038/sj.onc.1200986. PMID 9135077.
- Latinkic BV, O'Brien TP, Lau LF (1991). "Promoter function and structure of the growth factor-inducible immediate early gene cyr61". Nucleic Acids Res. 19 (12): 3261–3267. doi:10.1093/nar/19.12.3261. PMC 328320. PMID 2062642.
- Zhang Y, Diao Z, Su L, Sun H, Li R, Cui H, Hu Y (2010). "MicroRNA-155 contributes to preeclampsia by down-regulating CYR61". Am. J. Obstet. Gynecol. 202 (5): 466–467. doi:10.1016/j.ajog.2010.01.057. PMID 20452491.
- O'Brien TP, Yang GP, Sanders L, Lau LF (1990). "Expression of cyr61, a growth factor-inducible immediate-early gene". Mol. Cell. Biol. 10 (7): 3569–3577. doi:10.1128/MCB.10.7.3569. PMC 360792. PMID 2355916.
- Kireeva ML, Lam SC, Lau LF (1998). "Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin αvβ3". J. Biol. Chem. 273 (5): 3090–3096. doi:10.1074/jbc.273.5.3090. PMID 9446626.
- Chen N, Chen CC, Lau LF (2000). "Adhesion of human skin fibroblasts to Cyr61 is mediated through integrin α6β1 and cell surface heparan sulfate proteoglycans". J. Biol. Chem. 275 (32): 24953–24961. doi:10.1074/jbc.M003040200. PMID 10821835.
- Grzeszkiewicz TM, Lindner V, Chen N, Lam SC, Lau LF (2002). "The angiogenic factor CYR61 supports vascular smooth muscle cell adhesion and stimulates chemotaxis through integrin α6β1 and cell surface heparan sulfate proteoglycans". Endocrinology. 143 (4): 1441–1450. doi:10.1210/en.143.4.1441. PMID 11897702.
- Jedsadayanmata A, Chen CC, Kireeva ML, Lau LF, Lam SC (1999). "Activation-dependent adhesion of human platelets to Cyr61 and Fisp12/Mouse connective tissue growth factor is mediated through integrin αIIbβ3". J. Biol. Chem. 274 (34): 24321–24327. doi:10.1074/jbc.274.34.24321. PMID 10446209.
- Schober JM, Chen N, Grzeszkiewicz TM, Emeson EE, Ugarova TP, Ye RD, Lau LF, Lam SC, Lam SC (2002). "Identification of integrin αMβ2 as an adhesion receptor on peripheral blood moncytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2), immediate-early gene products expressed in atherosclerotic lesions". Blood. 99 (12): 4457–4465. doi:10.1182/blood.V99.12.4457. PMID 12036876.
- Bai T, Chen CC, Lau LF (2010). "The matricellular protein CCN1 activates a pro-inflammatory genetic program in murine macrophages". J. Immunol. 184 (6): 3223–3232. doi:10.4049/jimmunol.0902792. PMC 2832719. PMID 20164416.
- Yakubenko VP, Yadav SP, Ugarova TP (2006). "Integrin αDβ2, an adhesion receptor up-regulated on macrophage foam cells, exhibits multiligand-binding properties". Blood. 107 (4): 1643–1650. doi:10.1182/blood-2005-06-2509. PMC 1367263. PMID 16239428.
- Todorovic V, Chen CC, Hay N, Lau LF (2005). "The matrix protein CCN1 (CYR61) induces apoptosis in fibroblasts". J. Cell Biol. 171 (3): 559–568. doi:10.1083/jcb.200504015. PMC 1626352. PMID 16275757.
- Chen CC, Young JL, Monzon RI, Chen N, Todorovic V, Lau LF (2007). "Cytotoxicity of TNFα is regulated by Integrin-Mediated Matrix Signaling". EMBO J. 26 (5): 1257–1267. doi:10.1038/sj.emboj.7601596. PMC 1817641. PMID 17318182.
- Leu SJ, Lam SC, Lau LF (2002). "Proangiogenic activities of CYR61 (CCN1) mediated through integrins αvβ3 and α6β1 in human umbilical vein endothelial cells". J. Biol. Chem. 277 (48): 46248–46255. doi:10.1074/jbc.M209288200. PMID 12364323.
- Jun JI, Lau LF (2010). "The Matricellular Protein CCN1/CYR61 Induces Fibroblast Senescence and Restricts Fibrosis in Cutaneous Wound Healing". Nat. Cell Biol. 12 (7): 676–685. doi:10.1038/ncb2070. PMC 2919364. PMID 20526329.
- Chen CC, Chen N, Lau LF (2001). "The angiogenic factors Cyr61 and CTGF induce adhesive signaling in primary human skin fibroblasts". J. Biol. Chem. 276 (13): 10443–10452. doi:10.1074/jbc.M008087200. PMID 11120741.
- Emre, Yalin; Irla, Magali; Dunand-Sauthier, Isabelle; Ballet, Romain; Meguenani, Mehdi; Jemelin, Stephane; Vesin, Christian; Reith, Walter; Imhof, Beat A. (2013-11-27). "Thymic epithelial cell expansion through matricellular protein CYR61 boosts progenitor homing and T-cell output". Nature Communications. 4: 2842. doi:10.1038/ncomms3842. PMID 24280864.
- Babic AM, Kireeva ML, Kolesnikova TV, Lau LF (1998). "CYR61, product of a growth factor-inducible immediate-early gene, promotes angiogenesis and tumor growth". Proc. Natl. Acad. Sci. U.S.A. 95 (11): 6355–6360. doi:10.1073/pnas.95.11.6355. PMC 27701. PMID 9600969.
- Fataccioli V, Abergel V, Wingertsmann L, Neuville P, Spitz E, Adnot S, Calenda V, Teiger E (2002). "Stimulation of angiogenesis by cyr61 gene: a new therapeutic candidate". Hum. Gene Ther. 13 (12): 1461–1470. doi:10.1089/10430340260185094. PMID 12215267.
- Wong M, Kireeva ML, Kolesnikova TV, Lau LF (1997). "Cyr61, product of a growth factor-inducible immediate-early gene, regulates chondrogenesis in mouse limb bud mesenchymal cells". Dev. Biol. 192 (2): 492–508. doi:10.1006/dbio.1997.8766. PMID 9441684.
- Si W, Kang Q, Luu HH, Park JK, Luo Q, Song WX, Jiang W, Luo X, Li X (2006). "CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells". Mol. Cell. Biol. 26 (8): 2955–2964. doi:10.1128/MCB.26.8.2955-2964.2006. PMC 1446962. PMID 16581771.
- Crockett JC, Schutze N, Tosh D, Jatzke S, Duthie A, Jakob F, Rogers MJ (2007). "The matricellular protein CYR61 inhibits osteoclastogenesis by a mechanism independent of αvβ3 and αvβ5". Endocrinology. 148 (12): 5761–5768. doi:10.1210/en.2007-0473. PMID 17823253.
- Su JL, Chiou J, Tang CH, Zhao M, Tsai CH, Chen PS, Chang YW, Chien MH, Peng CY (2010). "CYR61 regulates BMP-2-dependent osteoblast differentiation through the αvβ3 integrin/integrin-linked kinase/ERK pathway". J. Biol. Chem. 285 (41): 31325–31336. doi:10.1074/jbc.M109.087122. PMC 2951207. PMID 20675382.
- O'Brian TP, Lau LF (1992). "Expression of the Growth Factor-Inducible Immediate Early Gene cyr61 Correlates with Chondrogenesis During Mouse Embryonic Development". Cell Growth & Differentiation. 3 (9): 645–654. PMID 1419914.
- Latinkic BV, Mo FE, Greenspan JA, Copeland NG, Gilbert DJ, Jenkins NA, Ross SR, Lau LF (2001). "Promoter Function of the Angiogenic Inducer Cyr61 Gene in Transgenic Mice: Tissue Specificity, Inducibility During Wound Healing, and Role of the Serum Response Element". Endocrinology. 142 (6): 2549–2557. doi:10.1210/en.142.6.2549. PMID 11356704.
- Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF (2002). "CYR61 (CCN1) Is Essential for Placental Development and Vascular Integrity". Mol. Cell. Biol. 22 (24): 8709–8720. doi:10.1128/MCB.22.24.8709-8720.2002. PMC 139880. PMID 12446788.
- Mo FE, Lau LF (2006). "The matricellular protein CCN1 is essential for cardiac development". Circ. Res. 99 (9): 961–969. doi:10.1161/01.RES.0000248426.35019.89. PMC 1626492. PMID 17023674.
- Latinkic BV, Mercurio S, Bennett B, Hirst EM, Xu Q, Lau LF, Mohun TJ, Smith JC (2003). "Xenopus Cyr61 regulates gastrulation movements and modulates Wnt signalling". Development. 130 (11): 2429–2441. doi:10.1242/dev.00449. PMID 12702657.
- Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008). "Wound repair and regeneration". Nature. 453 (7193): 314–321. doi:10.1038/nature07039. PMID 18480812.
- Wynn TA (2008). "Cellular and molecular mechanisms of fibrosis". The Journal of Pathology. 214 (2): 199–210. doi:10.1002/path.2277. PMC 2693329. PMID 18161745.
- Jun JI, Lau LF (2010). "Cellular senescence controls fibrosis in wound healing". Aging. 2 (9): 627–631. doi:10.18632/aging.100201. PMC 2984611. PMID 20930261.
- Hilfiker-Kleiner D, Kaminski K, Kaminska A, Fuchs M, Klein G, Podewski E, Grote K, Kiian I, Wollert KC (2004). "Regulation of proangiogenic factor CCN1 in cardiac muscle: impact of ischemia, pressure overload, and neurohumoral activation". Circulation. 109 (18): 2227–2233. doi:10.1161/01.CIR.0000127952.90508.9D. PMID 15117851.
- Hadjiargyrou M, Ahrens W, Rubin CT (2000). "Temporal expression of the chondrogenic and angiogenic growth factor CYR61 during fracture repair". J. Bone Miner. Res. 15 (6): 1014–1023. doi:10.1359/jbmr.2000.15.6.1014. PMID 10841170.
- Nakata E, Nakanishi T, Kawai A, Asaumi K, Yamaai T, Asano M, Nishida T, Mitani S, Inoue H (2002). "Expression of connective tissue growth factor/hypertrophic chondrocyte-specific gene product 24 (CTGF/Hcs24) during fracture healing". Bone. 31 (4): 441–447. doi:10.1016/S8756-3282(02)00846-3. PMID 12398938.
- Athanasopoulos AN, Schneider D, Keiper T, Alt V, Pendurthi UR, Liegibel UM, Sommer U, Nawroth PP, Kasperk C (2007). "Vascular endothelial growth factor (VEGF)-induced up-regulation of CCN1 in osteoblasts mediates proangiogenic activities in endothelial cells and promotes fracture healing". J. Biol. Chem. 282 (37): 26746–26753. doi:10.1074/jbc.M705200200. PMC 2831223. PMID 17626014.
- Sawai K, Mukoyama M, Mori K, Kasahara M, Koshikawa M, Yokoi H, Yoshioka T, Ogawa Y, Sugawara A, Nishiyama H, Yamada S, Kuwahara T, Saleem MA, Shiota K, Ogawa O, Miyazato M, Kangawa K, Nakao K (October 2007). "Expression of CCN1 (CYR61) in developing, normal, and diseased human kidney". Am. J. Physiol. Renal Physiol. 293 (4): F1363–72. doi:10.1152/ajprenal.00205.2007. PMID 17699553.
- Juric V, Chen CC, Lau LF (2009). "Fas-Mediated Apoptosis is Regulated by the Extracellular Matrix Protein CCN1 (CYR61) in vitro and in vivo". Mol. Cell. Biol. 29 (12): 3266–3279. doi:10.1128/MCB.00064-09. PMC 2698731. PMID 19364818.
- Franzen CA, Chen CC, Todorovic V, Juric V, Monzon RI, Lau LF (2009). "The Matrix Protein CCN1 is Critical for Prostate Carcinoma Cell Proliferation and TRAIL-Induced Apoptosis". Mol. Cancer Res. 7 (7): 1045–1055. doi:10.1158/1541-7786.MCR-09-0017. PMC 2712585. PMID 19584265.
- Koon HW, Zhao D, Xu H, Bowe C, Moss A, Moyer MP, Pothoulakis C (2008). "Substance P-mediated expression of the pro-angiogenic factor CCN1 modulates the course of colitis". Am. J. Pathol. 173 (2): 400–410. doi:10.2353/ajpath.2008.080222. PMC 2475777. PMID 18599605.
- Imhof, Beat A.; Jemelin, Stephane; Ballet, Romain; Vesin, Christian; Schapira, Marco; Karaca, Melis; Emre, Yalin (2016-08-01). "CCN1/CYR61-mediated meticulous patrolling by Ly6Clow monocytes fuels vascular inflammation". Proceedings of the National Academy of Sciences. 113 (33): E4847–E4856. doi:10.1073/pnas.1607710113. ISSN 0027-8424. PMC 4995973. PMID 27482114.
- Kok SH, Hou KL, Hong CY, Wang JS, Liang PC, Chang CC, Hsiao M, Yang H, Lai EH, Lin SK (April 2011). "Simvastatin inhibits cytokine-stimulated Cyr61 expression in osteoblastic cells: a therapeutic benefit for arthritis". Arthritis Rheum. 63 (4): 1010–20. doi:10.1002/art.27433. PMID 20191585.
- Chijiiwa M, Mochizuki S, Kimura T, Abe H, Tanaka Y, Fujii Y, Shimizu H, Enomoto H, Toyama Y, Okada Y (June 2015). "CCN1 (Cyr61) Is Overexpressed in Human Osteoarthritic Cartilage and Inhibits ADAMTS-4 (Aggrecanase 1) Activity". Arthritis & Rheumatology. 67 (6): 1557–67. doi:10.1002/art.39078. PMID 25709087.
- Hilfiker A, Hilfiker-Kleiner D, Fuchs M, Kaminski K, Lichtenberg A, Rothkotter HJ, Schieffer B, Drexler H (2002). "Expression of CYR61, an angiogenic immediate early gene, in arteriosclerosis and its regulation by angiotensin II". Circulation. 106 (2): 254–260. doi:10.1161/01.CIR.0000021426.87274.62. PMID 12105167.
- Sigala F, Georgopoulos S, Papalambros E, Chasiotis D, Vourliotakis G, Niforou A, Kotsinas A, Kavantzas N, Patsouris E (2006). "Heregulin, cysteine rich-61 and matrix metalloproteinase 9 expression in human carotid atherosclerotic plaques: relationship with clinical data". Eur. J. Vasc. Endovasc. Surg. 32 (3): 238–245. doi:10.1016/j.ejvs.2006.01.026. PMID 16774841.
- Lee HY, Chung JW, Youn SW, Kim JY, Park KW, Koo BK, Oh BH, Park YB, Chaqour B (2007). "Forkhead transcription factor FOXO3a is a negative regulator of angiogenic immediate early gene CYR61, leading to inhibition of vascular smooth muscle cell proliferation and neointimal hyperplasia". Circ. Res. 100 (3): 372–380. doi:10.1161/01.RES.0000257945.97958.77. PMID 17234971.
- Matsumae H, Yoshida Y, Ono K, Togi K, Inoue K, Furukawa Y, Nakashima Y, Kojima Y, Nobuyoshi M (2008). "CCN1 Knockdown Suppresses Neointimal Hyperplasia in a Rat Artery Balloon Injury Model". Arterioscler. Thromb. Vasc. Biol. 28 (6): 1077–1083. doi:10.1161/ATVBAHA.108.162362. PMID 18388330.
- Hasan A, Pokeza N, Shaw L, Lee HS, Lazzaro D, Chintala H, Rosenbaum D, Grant MB, Chaqour B (March 2011). "The matricellular protein cysteine-rich protein 61 (CCN1/Cyr61) enhances physiological adaptation of retinal vessels and reduces pathological neovascularization associated with ischemic retinopathy". J. Biol. Chem. 286 (11): 9542–54. doi:10.1074/jbc.M110.198689. PMC 3059032. PMID 21212276.
- Folkman J (2006). "Angiogenesis". Annu. Rev. Med. 57: 1–18. doi:10.1146/annurev.med.57.121304.131306. PMID 16409133.
- Monnier Y, Farmer P, Bieler G, Imaizumi N, Sengstag T, Alghisi GC, Stehle JC, Ciarloni L, Blant S (2008). "CYR61 and αVβ5 Integrin Cooperate to Promote Invasion and Metastasis of Tumors Growing in Preirradiated Stroma". Cancer Res. 68 (18): 7323–7331. doi:10.1158/0008-5472.CAN-08-0841. PMID 18794119.
- Sun ZJ, Wang Y, Cai Z, Chen PP, Tong XJ, Xie D (2008). "Involvement of Cyr61 in growth, migration, and metastasis of prostate cancer cells". Br. J. Cancer. 99 (10): 1656–1667. doi:10.1038/sj.bjc.6604712. PMC 2584944. PMID 18941464.
- Kassis JN, Virador VM, Guancial EA, Kimm D, Ho AS, Mishra M, Chuang EY, Cook J, Gius D (2009). "Genomic and phenotypic analysis reveals a key role for CCN1 (CYR61) in BAG3-modulated adhesion and invasion". J. Pathol. 218 (4): 495–504. doi:10.1002/path.2557. PMC 7316387. PMID 19402132.
- Haque I, Mehta S, Majumder M, Dhar K, De A, McGregor D, Van Veldhuizen PJ, Banerjee SK, Banerjee S (2011). "Cyr61/CCN1 signaling is critical for epithelial-mesenchymal transition and stemness and promotes pancreatic carcinogenesis". Mol. Cancer. 10: 8. doi:10.1186/1476-4598-10-8. PMC 3027193. PMID 21232118.
- Xie D, Miller CW, O'Kelly J, Nakachi K, Sakashita A, Said JW, Gornbein J, Koeffler HP (2001). "Breast cancer. Cyr61 is overexpressed, estrogen-inducible, and associated with more advanced disease". J. Biol. Chem. 276 (17): 14187–14194. doi:10.1074/jbc.M009755200. PMID 11297518.
- Gery S, Xie D, Yin D, Gabra H, Miller C, Wang H, Scott D, Yi WS, Popoviciu ML (2005). "Ovarian carcinomas: CCN genes are aberrantly expressed and CCN1 promotes proliferation of these cells". Clin. Cancer Res. 11 (20): 7243–7254. doi:10.1158/1078-0432.CCR-05-0231. PMID 16243794.
- Kok SH, Chang HH, Tsai JY, Hung HC, Lin CY, Chiang CP, Liu CM, Kuo MY (2010). "Expression of Cyr61 (CCN1) in human oral squamous cell carcinoma: An independent marker for poor prognosis". Head Neck. 32 (12): 1665–1673. doi:10.1002/hed.21381. PMID 20848406.
- Tsai MS, Hornby AE, Lakins J, Lupu R (2000). "Expression and function of CYR61, an angiogenic factor, in breast cancer cell lines and tumor biopsies". Cancer Res. 60 (20): 5603–5607. PMID 11059746.
- Xie D, Nakachi K, Wang H, Elashoff R, Koeffler HP (2001). "Elevated levels of connective tissue growth factor, WISP-1, and CYR61 in primary breast cancers associated with more advanced features". Cancer Res. 61 (24): 8917–8923. PMID 11751417.
- Jiang WG, Watkins G, Fodstad O, Douglas-Jones A, Mokbel K, Mansel RE (2004). "Differential expression of the CCN family members Cyr61, CTGF and Nov in human breast cancer". Endocr. Relat. Cancer. 11 (4): 781–791. doi:10.1677/erc.1.00825. PMID 15613452.
- O'Kelly J, Chung A, Lemp N, Chumakova K, Yin D, Wang HJ, Said J, Gui D, Miller CW (2008). "Functional domains of CCN1 (Cyr61) regulate breast cancer progression". Int. J. Oncol. 33 (1): 59–67. doi:10.3892/ijo.33.1.59. PMID 18575751.
- D'Antonio KB, Toubaji A, Albadine R, Mondul AM, Platz EA, Netto GJ, Getzenberg RH (2010). "Extracellular matrix associated protein CYR61 is linked to prostate cancer development". J. Urol. 183 (4): 1604–1610. doi:10.1016/j.juro.2009.12.006. PMC 3349619. PMID 20172544.
- Goodwin CR, Lal B, Zhou X, Ho S, Xia S, Taeger A, Murray J, Laterra J (2010). "Cyr61 mediates hepatocyte growth factor-dependent tumor cell growth, migration, and Akt activation". Cancer Res. 70 (7): 2932–2941. doi:10.1158/0008-5472.CAN-09-3570. PMC 2848876. PMID 20233866.
- Lin MT, Zuon CY, Chang CC, Chen ST, Chen CP, Lin BR, Wang MY, Jeng YM, Chang KJ (2005). "Cyr61 induces gastric cancer cell motility/invasion via activation of the integrin/nuclear factor-κB/cyclooxygenase-2 signaling pathway". Clin. Cancer Res. 11 (16): 5809–5820. doi:10.1158/1078-0432.CCR-04-2639. PMID 16115920.
- Chen CC, Lau LF (2010). "Deadly liaisons: fatal attraction between CCN matricellular proteins and the tumor necrosis factor family of cytokines". J. Cell Commun. Signal. 4 (1): 63–69. doi:10.1007/s12079-009-0080-4. PMC 2821476. PMID 19898959.
- Schmitt CA (2003). "Senescence, apoptosis and therapy--cutting the lifelines of cancer". Nature Reviews Cancer. 3 (4): 286–295. doi:10.1038/nrc1044. PMID 12671667.
- Sakamoto S, Yokoyama M, Aoki M, Suzuki K, Kakehi Y, Saito Y (2004). "Induction and function of CYR61 (CCN1) in prostatic stromal and epithelial cells: CYR61 is required for prostatic cell proliferation". Prostate. 61 (4): 305–317. doi:10.1002/pros.20098. PMID 15389821.
- Tong X, Xie D, O'Kelly J, Miller CW, Muller-Tidow C, Koeffler HP (2001). "Cyr61, a member of CCN family, is a tumor suppressor in non-small cell lung cancer". J. Biol. Chem. 276 (50): 47709–47714. doi:10.1074/jbc.M107878200. PMID 11598125.
- Chien W, Kumagai T, Miller CW, Desmond JC, Frank JM, Said JW, Koeffler HP (2004). "Cyr61 suppresses growth of human endometrial cancer cells". J. Biol. Chem. 279 (51): 53087–53096. doi:10.1074/jbc.M410254200. PMID 15471875.
- Dobroff AS, Wang H, Melnikova VO, Villares GJ, Zigler M, Huang L, Bar-Eli M (2009). "Silencing cAMP-response element binding protein (CREB) identifies cysteine-rich protein 61 (CYR61) as a tumor suppressor gene in melanoma". J. Biol. Chem. 284 (38): 26194–26206. doi:10.1074/jbc.M109.019836. PMC 2758018. PMID 19632997.