Candidate phyla radiation
Candidate phyla radiation (also referred to as CPR group) is a large evolutionary radiation of bacterial lineages whose members are mostly uncultivated and only known from metagenomics and single cell sequencing. They have been described as nanobacteria or ultra-small bacteria due to their reduced size (nanometric) compared to other bacteria. Originally, it has been suggested that CPR represents over 15% of all bacterial diversity and may consist of more than 70 different phyla.[1] However, a recently proposed standardized bacterial taxonomy based on relative evolutionary divergence found that CPR represents a single phylum.[2] CPR lineages are generally characterized as having small genomes and lacking several biosynthetic pathways and ribosomal proteins. This has led to the speculation that they are likely obligate symbionts.[3][4]
| Candidate phyla radiation | |
|---|---|
 | |
| Representation of a bacterium of this phylum. | |
| Scientific classification | |
| Domain: | Bacteria |
| (unranked): | Bacteria candidate phyla |
| Infrakingdom: | Candidate phyla radiation |
Earlier work proposed a superphylum called Patescibacteria which encompassed several phyla later attributed to the CPR group.[5] Therefore, Patescibacteria and CPR are often used as synonyms.[6]
Characteristics
Although there are a few exceptions, members of the candidate phyla radiation generally lack several biosynthetic pathways for several amino acids and nucleotides. To date, there has been no genomic evidence that indicates that they are capable of producing the lipids essential for cell envelope formation.[4] Additionally, they tend to lack complete TCA cycles and electron transport chain complexes, including ATP synthase. This lack of several important pathways found in most free-living prokaryotes indicates that the candidate phyla radiation is composed of obligate fermentative symbionts.[7]
Furthermore, CPR members have unique ribosomal features. While the members of CPR are generally uncultivable, and therefore missed in culture-dependent methods, they are also often missed in culture-independent studies that rely on 16S rRNA sequences. Their rRNA genes appear to encode proteins and have self-splicing introns, features that are rarely seen in bacteria, although they have previously been reported.[8] Owing to these introns, members of CPR are not detected in 16S-dependent methods. Additionally, all CPR members are missing the L30 ribosomal protein, a trait that is often seen in symbionts.[7]
Many of its characteristics are similar or analogous to those of ultra-small archaea (DPANN).[4]
Phylogeny
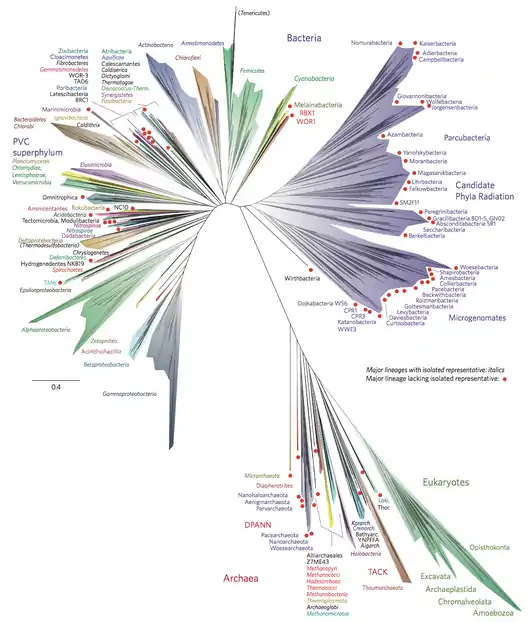
Candidate phyla radiation is the first clade to separate from bacteria according to some phylogenetic analyses based on ribosomal proteins, 16S rRNA, and protein family presence. These phylogenetic analyses have found the following phylogeny between phyla and superphyla. The superphyla are shown in bold.[4][3]
| Bacteria |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Alternatively, it has been proposed that the CPR group could belong to Terrabacteria being more closely related to Chloroflexi. An alternative location for CPR in the phylogenetic tree is as follows.[9]
| Bacteria |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Provisional taxonomy
Because many CPR members are uncultivable, they cannot be formally put into the bacterial taxonomy, but a number of provisional, or Candidatus, names have been generally agreed on.[5] As of 2017, two superphyla are generally recognized under CPR, Parcubacteria and Microgenomates.[1] The Phyla under CPR include:
- Unranked Parcubacteria Cluster
- Superphylum Parcubacteria
- Phylum Kerfeldbacteria
- Phylum Doudnabacteria – forms a monophyletic group with the paraphyletic Parcubacteria
- Phylum KAZAN
- Phylum CPR2
- Phylum Berkelbacteria (ACD58)
- Phylum Gracilibacteria
- Phylum Saccharibacteria (TM7) – one member, TM7x, cultivated
- Phylum Peregrinibacteria
- Phylum Peribacteria
- Superphylum Parcubacteria
- Unranked Microgenomates Cluster
- Superphylum Microgenomates
- Phylum Woekebacteria
- Phylum Beckwithbacteria
- Phylum Pacebacteria
- Phylum Chisholmbacteria
- Phylum Collierbacteria
- Phylum Katanobacteria (WWE3)
- Superphylum Microgenomates
The current phylogeny is based on ribosomal proteins (Hug et al., 2016).[3] Other approaches, including protein family existence and 16S ribosomal RNA, produce similar results at lower resolutions.[10][1]
See also
- Bacterial phyla § Uncultivated phyla and metagenomics for some of the phyla in CPR.
References
- Danczak RE, Johnston MD, Kenah C, Slattery M, Wrighton KC, Wilkins MJ (September 2017). "Members of the candidate phyla radiation are functionally differentiated by carbon- and nitrogen-cycling capabilities". Microbiome. 5 (1): 112. doi:10.1186/s40168-017-0331-1. PMC 5581439. PMID 28865481.
- Parks, Donovan; Chuvochina, Maria; Waite, David; Rinke, Christian; Skarshewski, Adam; Chaumeil, Pierre-Alain; Hugenholtz, Philip (27 August 2018). "A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life". Nature Biotechnology. 36 (10): 996–1004. doi:10.1038/nbt.4229. PMID 30148503. S2CID 52093100. Retrieved 13 January 2021.
- Hug LA, Baker BJ, Anantharaman K, Brown CT, Probst AJ, Castelle CJ, et al. (April 2016). "A new view of the tree of life". Nature Microbiology. 1 (5): 16048. doi:10.1038/nmicrobiol.2016.48. PMID 27572647.
- Castelle CJ, Banfield JF (March 2018). "Major New Microbial Groups Expand Diversity and Alter our Understanding of the Tree of Life". Cell. 172 (6): 1181–1197. doi:10.1016/j.cell.2018.02.016. PMID 29522741.
- Rinke C; et al. (2013). "Insights into the phylogeny and coding potential of microbial dark matter". Nature. 499 (7459): 431–7. Bibcode:2013Natur.499..431R. doi:10.1038/nature12352. PMID 23851394.
- Jacob P. Beam, Eric D. Becraft, Julia M. Brown, Frederik Schulz, Jessica K. Jarett, Oliver Bezuidt, Nicole J. Poulton, Kayla Clark, Peter F. Dunfield, Nikolai V. Ravin, John R. Spear, Brian P. Hedlund, Konstantinos A. Kormas, Stefan M. Sievert, Mostafa S. Elshahed, Hazel A. Barton, Matthew B. Stott, Jonathan A. Eisen, Duane P. Moser, Tullis C. Onstott, Tanja Woyke, Ramunas Stepanauskas: Ancestral Absence of Electron Transport Chains in Patescibacteria and DPANN, in: Frontiers in Microbiology, Band 11, 2020, doi:10.3389/fmicb.2020.01848
- Brown CT, Hug LA, Thomas BC, Sharon I, Castelle CJ, Singh A, et al. (July 2015). "Unusual biology across a group comprising more than 15% of domain Bacteria". Nature. 523 (7559): 208–11. Bibcode:2015Natur.523..208B. doi:10.1038/nature14486. OSTI 1512215. PMID 26083755. S2CID 4397558.
- Belfort M, Reaban ME, Coetzee T, Dalgaard JZ (July 1995). "Prokaryotic introns and inteins: a panoply of form and function". Journal of Bacteriology. 177 (14): 3897–903. doi:10.1128/jb.177.14.3897-3903.1995. PMC 177115. PMID 7608058.
- Coleman, Gareth A.; Davín, Adrián A.; Mahendrarajah, Tara; Spang, Anja; Hugenholtz, Philip; Szöllősi, Gergely J.; Williams, Tom A. (15 July 2020). "A rooted phylogeny resolves early bacterial evolution". BiorXiv Preprint. bioRxiv 10.1101/2020.07.15.205187. doi:10.1101/2020.07.15.205187. S2CID 220602262.
- Méheust, Raphaël; Burstein, David; Castelle, Cindy J.; Banfield, Jillian F. (13 September 2019). "The distinction of CPR bacteria from other bacteria based on protein family content". Nature Communications. 10 (1): 4173. Bibcode:2019NatCo..10.4173M. doi:10.1038/s41467-019-12171-z. PMC 6744442. PMID 31519891.
External links
- Most of the Tree of Life is a Complete Mystery. We know certain branches exist, but we have never seen the organisms that perch there. by Ed Yong, April 12, 2016, atlantic.com.
- Ultra-Small, Parasitic Bacteria Found in Groundwater, Dogs, Cats — And You; on: SciTechDaily; July 21, 2020; source: Forsyth Institute
- Jeffrey S. McLean, Batbileg Bor, Kristopher A. Kerns, Kelly Wrighton, Wenyuan Shi, Xuesong He, et al.: Acquisition and Adaptation of Ultra-small Parasitic Reduced Genome Bacteria to Mammalian Hosts; in: CellRep 32, 107939; July 21, 2020; doi:10.1016/j.celrep.2020.107939
- Jeffrey S. McLean, Batbileg Bor, Thao T. To, Quanhui Liu, Kristopher A. Kerns, Lindsey Solden, Kelly Wrighton, Xuesong He, Wenyuan Shi: Evidence of independent acquisition and adaption of ultra-small bacteria to human hosts across the highly diverse yet reduced genomes of the phylum Saccharibacteria; on: bioRxiv; February 2, 2018; doi:10.1101/258137 (PrePrint)
- Bokhari, RH; Amirjan, N; Jeong, H; Kim, KM; Caetano-Anollés, G; Nasir, A (1 March 2020). "Bacterial Origin and Reductive Evolution of the CPR Group". Genome Biology and Evolution. 12 (3): 103–121. doi:10.1093/gbe/evaa024. PMC 7093835. PMID 32031619.