Bacterial phyla
Bacterial phyla constitute the major lineages of the domain Bacteria. While the exact definition of a bacterial phylum is debated, a popular definition is that a bacterial phylum is a monophyletic lineage of bacteria whose 16S rRNA genes share a pairwise sequence identity of ~75% or less with those of the members of other bacterial phyla.[2]
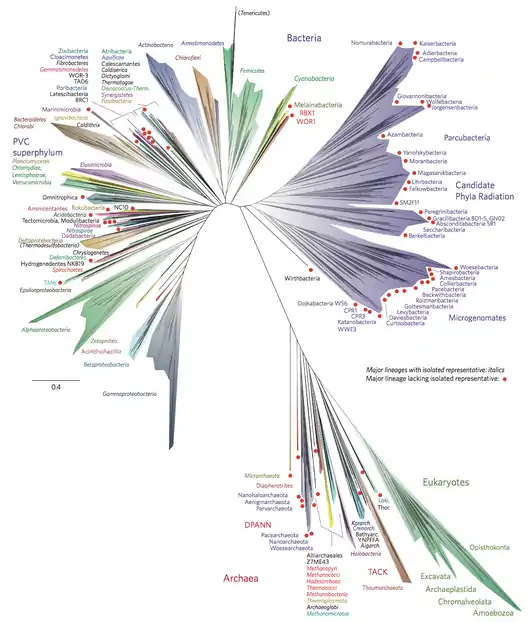
It has been estimated that ~1,300 bacterial phyla exist.[2] As of May 2020, 41 bacterial phyla are formally accepted by the LPSN,[3] 89 bacterial phyla are recognized on the Silva database, dozens more have been proposed,[4][5] and hundreds likely remain to be discovered.[2] As of 2017, approximately 72% of widely recognized bacterial phyla were candidate phyla[6] (i.e. have no cultured representatives).
There are no fixed rules to the nomenclature of bacterial phyla. It was proposed that the suffix "-bacteria" be used for phyla.
List of bacterial phyla
The following is a list of bacterial phyla that have been proposed.
| Phylum | Alternative names | Group | Cultured representative | Notes |
|---|---|---|---|---|
| 10bav-F6[7] | No | |||
| Abawacabacteria[4][8] | RIF46 | CPR; Gracilibacteria-related CPR | No | |
| Abditibacteriota[9] | FBP | Yes[9] | ||
| Absconditabacteria[10][8] | SR1 | CPR; Gracilibacteria-related CPR | No | |
| ABY1[11] | OD1-ABY1[12] | CPR; Parcubacteria | No | |
| Acetothermia[13] | OP1 | |||
| Acidobacteria | Yes[14] | |||
| Actinobacteria | Terrabacteria | Yes[15] | ||
| Adlerbacteria[16][8] | CPR; Patescibacteria; Parcubacteria; Parcubacteria 4 | No | ||
| Aerophobota / Aerophobetes | CD12, BHI80-139 | |||
| Amesbacteria[16] | CPR; Patescibacteria;
Microgenomates |
No | ||
| Andersenbacteria[4] | RIF9 | CPR; Parcubacteria; Parcubacteria 4-related | No | |
| Armatimonadetes[13] | OP10 | Terrabacteria | Yes[17] | |
| Aminicenantes[13] | OP8 | |||
| AncK6[7] | ||||
| Apal-E12[7] | ||||
| Atribacteria[13] | OP9, JS1 | No | ||
| Aquificae | ||||
| Azambacteria i[16][8] | CPR; Patescibacteria; Parcubacteria; unclassified Parcubacteria | No | splitted by Anantharaman et al. … | |
| Azambacteria ii[16][8] | CPR; Patescibacteria; Parcubacteria; unclassified Parcubacteria | No | … (Oct 2016) as being polyphyletic | |
| Bacteroidetes | FCB group | Yes | ||
| Balneolaeota[18] | Yes | |||
| Bdellovibrionota | ||||
| Beckwithbacteria[16] | CPR; Patescibacteria; Microgenomates | No | ||
| Berkelbacteria[19][8] | ACD58 | CPR; Saccharibacteria-related CPR | No | |
| BHI80-139[7] | ||||
| Blackburnbacteria[4] | RIF35 | CPR; Microgenomates | No | |
| Brennerbacteria[4][8] | RIF18 | CPR; Parcubacteria; Parcubacteria 3 | No | |
| Brownbacteria[20] | CPR; Parcubacteria; unclassified Parcubacteria | No | ||
| Buchananbacteria[4][8] | RIF37; Parcubacteria; Parcubacteria 1 | CPR | No | |
| Caldiserica[13] | OP5[21] | FCB group | Yes[22] | |
| Calditrichaeota[23] | Caldithrix | FCB group[24] | ||
| Calescamantes | EM19 | |||
| Campbellbacteria[16][8] | CPR; Patescibacteria; Parcubacteria; Parcubacteria 4 | No | seem to be polyphyletic: two clades | |
| Chlamydiae[25] | PVC group | |||
| Chlorobi | FCB group | |||
| Chloroflexi | Terrabacteria | |||
| Chisholmbacteria[4] | RIF36 | CPR; Microgenomates | No | |
| Chrysiogenetes | ||||
| Cloacimonetes[26] | WWE1 | FCB group[24] | ||
| Coatesbacteria[4] | RIF8 | No | ||
| Collierbacteria[16] | CPR; Patescibacteria; Microgenomates | No | ||
| Colwellbacteria[4][8] | RIF41 | CPR; Parcubacteria; Parcubacteria 3 | No | |
| Curtissbacteria[16] | CPR; Patescibacteria; Microgenomates | No | ||
| CPR-1[1] | CPR | No | ||
| CPR-3[1] | CPR | No | ||
| Cyanobacteria | Terrabacteria | |||
| Dadabacteria[27] | No | |||
| Daviesbacteria[16] | CPR; Patescibacteria; Microgenomates | No | ||
| Delphibacteria[6] | FCB group | No | ||
| Delongbacteria[4] | RIF26, H-178 | No | ||
| Deferribacteres | ||||
| Deinococcus–Thermus | Terrabacteria | |||
| Dependentiae[28] | TM6 | |||
| Dictyoglomi[29] | ||||
| Dojkabacteria[8] | WS6 | CPR; Microgenomates-related CPR | ||
| Dormibacteraeota[30] | AD3 | No | ||
| Doudnabacteria[16][8] | SM2F11 | CPR; Parcubacteria; Parcubacteria 1-related | No | |
| Edwardsbacteria[5][4] | RIF29, UBP-2 [31] | No | ||
| Eisenbacteria[4] | RIF28 | FCB group | No | |
| Elusimicrobia[13] | OP7, Termite Group 1 (TG1)[21] | Yes[32] | ||
| Eremiobacteraeota[33][30] | WPS-2, Palusbacterota[34] | No | ||
| Falkowbacteria[16][8] | CPR; Patescibacteria; Parcubacteria; Parcubacteria 1 | No | ||
| Fermentibacteria[35] | Hyd24-12 | No | ||
| Fertabacteria[6] | CPR; Gracilibacteria-related CPR | No | ||
| Fibrobacteres | FCB group | |||
| Firestonebacteria[4] | RIF1 | No | ||
| Fervidibacteria | OctSpa1-106 | |||
| Fischerbacteria[4] | RIF25 | No | ||
| Firmicutes | Terrabacteria | |||
| Fraserbacteria[4] | RIF31 | No | ||
| Fusobacteria | ||||
| Gemmatimonadetes[36] | FCB group[24] | Yes[36] | ||
| Glassbacteria[4] | RIF5 | No | ||
| Giovannonibacteria[16][8] | CPR; Patescibacteria; Parcubacteria; Parcubacteria 4-related | No | ||
| Gottesmanbacteria[16] | CPR; Patescibacteria; Microgenomates | No | ||
| Gracilibacteria[37][8] | GN02, BD1-5, SN-2 | CPR; Patescibacteria; Gracilibacteria-related CPR | No | |
| Gribaldobacteria[4][8] | CPR; Parcubacteria; Parcubacteria 2 | No | ||
| Handelsmanbacteria[4] | RIF27 | No | ||
| Harrisonbacteria[4][8] | RIF43 | CPR; Parcubacteria; Parcubacteria 3 | No | |
| Howlettbacteria[8] | CPR; Saccharibacteria-related CPR | No | ||
| Hugbacteria[20] | CPR; Parcubacteria; unclassified Parcubacteria | No | ||
| Hydrogenedentes | NKB19 | No | ||
| Ignavibacteria | ZB1 | FCB group | ||
| Jacksonbacteria[4][8] | RIF38 | CPR; Parcubacteria; Parcubacteria 1 | No | |
| Jorgensenbacteria[16][8] | CPR; Patescibacteria; Parcubacteria; Parcubacteria 3 | No | ||
| Kaiserbacteria[16][8] | CPR; Patescibacteria; Parcubacteria; Parcubacteria 4 | No | ||
| Katanobacteria[38][8] | WWE3 | CPR; Microgenomates-related | No | |
| Kazanbacteria[8][4] | CPR; Saccharibacteria-related CPR | No | ||
| Kerfeldbacteria[4][8] | RIF4 | CPR; Parcubacteria; Parcubacteria 1 | No | |
| Komeilibacteria[4][8] | RIF6 | CPR; Parcubacteria; Parcubacteria 1 | No | sometimes misspelled as "Komelilbacteria"[4] |
| Kryptonia[39] | No | |||
| KSB1 | No | |||
| Krumholzibacteriota[31] | ||||
| Kuenenbacteria[16][8] | CPR; Patescibacteria; Parcubacteria; Parcubacteria 1 | No | ||
| Lambdaproteobacteria[4] | RIF24 | Proteobacteria | No | |
| Latescibacteria | WS3 | FCB group[24] | No | |
| LCP-89[40] | ||||
| Lentisphaerae | vadinBE97 | PVC group | ||
| Levybacteria[16] | CPR; Patescibacteria; Microgenomates | No | ||
| Lindowbacteria[4] | RIF2 | CPR; Saccharibacteria-related CPR | No | |
| Liptonbacteria[4][8] | RIF42 | CPR; Parcubacteria; Parcubacteria 3 | No | |
| Lloydbacteria[4][8] | RIF45 | CPR; Parcubacteria; Parcubacteria 4 | No | |
| Magasanikbacteria[16][41][8] | CPR; Patescibacteria; Parcubacteria; Parcubacteria 1 | No | ||
| Margulisbacteria[4] | RIF30 | No | ||
| Marinimicrobia | SAR406, Marine Group A | FCB group[24] | Yes | |
| Melainabacteria[42] | No | |||
| Microgenomates[43] | OP11 | CPR; Patescibacteria | No | Superphylum |
| Modulibacteria[37][44] | KSB3, GN06 | No | ||
| Moranbacteria[16][8] | OD1-i[16] | CPR; Patescibacteria; Parcubacteria; unclassified Parcubacteria | No | |
| Muproteobacteria[4] | RIF23 | Proteobacteria | No | |
| NC10[45][11] | No | |||
| Nealsonbacteria[4][8] | RIF40 | CPR; Parcubacteria; Parcubacteria 2 | No | |
| Niyogibacteria[4] | RIF11 | CPR; Parcubacteria; Parcubacteria 4-related | No | |
| Nitrospinae[46] | ||||
| Nitrospirae | ||||
| Nomurabacteria[16][8] | CPR; Patescibacteria; Parcubacteria; Parcubacteria 1 | No | ||
| Omnitrophica[13] | OP3 | PVC group | No | |
| Pacebacteria[16] | CPR; Patescibacteria; Microgenomates | No | ||
| Parcubacteria[10] | OD1 | CPR | No | Superphylum |
| Parcubacteria 1[8] | CPR; Parcubacteria | No | ||
| Parcubacteria 2[8] | CPR; Parcubacteria | No | ||
| Parcubacteria 3[8] | CPR; Parcubacteria | No | ||
| Parcubacteria 4[8] | CPR; Parcubacteria | No | ||
| Parcunitrobacteria[47] | CPR; Parcubacteria; unclassified Parcubacteria[48] | No | Superphylum | |
| PAUC34f[49] | sponge‐associated unclassified lineage (SAUL) | FCB group | ||
| Peregrinibacteria[50][51][52][53][8] | PER | CPR; Gracilibacteria-related CPR | No | |
| Peribacteria[8] | CPR; Gracilibacteria-related CPR | No | ||
| Planctomycetes | PVC group | |||
| Poribacteria[54] | PVC group | |||
| Portnoybacteria[4] | RIF22 | CPR; Parcubacteria; Parcubacteria 4-related | No | |
| Proteobacteria | ||||
| Raymondbacteria[4] | RIF7 | No | ||
| Riflebacteria[4] | RIF32 | No | ||
| Roizmanbacteria[16] | CPR; Patescibacteria; Microgenomates | No | ||
| Rokubacteria[27] | No | |||
| Ryanbacteria[4][8] | RIF10 | CPR; Parcubacteria; Parcubacteri 4-related | No | |
| Saccharibacteria[28][8] | TM7 | CPR; Saccharibacteria-related CPR | Yes | |
| Saltatorellota[55] | ||||
| Schekmanbacteria[4] | RIF3 | Proteobacteria | No | |
| Shapirobacteria[16] | CPR; Patescibacteria; Microgenomates | No | ||
| Spechtbacteria[4][8] | RIF19 | CPR; Parcubacteria; Parcubacteria 2 | No | |
| Spirochaetes | ||||
| Staskawiczbacteria[4][8] | RIF20 | CPR; Parcubacteria; Parcubacteria 2 | No | |
| Sumerlaeota[56][57] | BRC1 | |||
| Sungbacteria[4][8] | RIF17 | CPR; Parcubacteria; Parcubacteria 4-related | No | |
| Synergistetes | ||||
| TA06[58] | No | |||
| Tagabacteria[4][8] | RIF12 | CPR; Parcubacteria; Parcubacteria 4-related | No | |
| Taylorbacteria[4][8] | RIF16 | CPR; Parcubacteria; Parcubacteria 4 | No | |
| Tectomicrobia[59] | ||||
| Tenericutes | ||||
| Terrybacteria[4][8] | RIF13 | CPR; Parcubacteria; Parcubacteria 2 | No | |
| Thermodesulfobacteria | ||||
| Thermomicrobia | ||||
| Thermotogae | OP2, EM3[21] | Yes[60] | ||
| Torobacteria[8] | CPR; Parcubacteria; unclssified Parcubacteria | No | ||
| UBP-1[5] | No | |||
| UBP-3[5] | No | |||
| UBP-4[5] | No | |||
| UBP-5[5] | No | |||
| UBP-6[5] | No | |||
| UBP-7[5] | No | |||
| UBP-8[5] | No | |||
| UBP-9[5] | No | |||
| UBP-10[5] | No | |||
| UBP-11[5] | No | |||
| UBP-12[5] | No | |||
| UBP-13[5] | No | |||
| UBP-14[5] | No | |||
| UBP-15[5] | No | |||
| UBP-16[5] | No | |||
| UBP-17[5] | No | |||
| Uhrbacteria[16][8] | CPR; Patescibacteria; Parcubacteria; Parcubacteria 1 | No | seem to be polyphyletic: two clades | |
| Veblenbacteria[4] | RIF39 | CPR; Parcubacteria; Parcubacteria 1-related | No | |
| Verrucomicrobia | PVC group | |||
| Vogelbacteria[4][8] | RIF14 | CPR; Parcubacteria; Parcubacteria 4 | No | |
| Wallbacteria[4] | RIF33 | No | ||
| Wildermuthbacteria[4][8] | RIF21 | CPR; Parcubacteria; Parcubacteria 2 | No | |
| Wirthbacteria[61] | CPR-related bacteria | No | ||
| Woesebacteria[16] | CPR; Patescibacteria; Microgenomates | No | ||
| Wolfebacteria[16][8] | CPR; Patescibacteria; Parcubacteria; Parcubacteria 3 | No | ||
| Woykebacteria[4][20] | RIF34 | CPR; Microgenomates | No | |
| WOR-1[58] | No | |||
| WOR-2[58] | No | |||
| WOR-3[58] | No | |||
| Yanofskybacteria[16][8] | CPR; Patescibacteria; Parcubacteria; unclassified Parcubacteria | No | ||
| Yonathbacteria[4][8] | RIF44 | CPR; Parcubacteria; Parcubacteria 4 | No | |
| Zambryskibacteria[4][8] | RIF15 | CPR; Parcubacteria; Parcubacteria 4 | No | |
| ZB2 | OD1-ZB2[12] | CPR; Parcubacteria | No | |
| Zixibacteria[62] | FCB group | No |
Supergroups
Despite the unclear branching order for most bacterial phyla, several groups of phyla consistently cluster together and are referred to as supergroups or superphyla. In some instances, bacterial clades clearly consistently cluster together but it is unclear what to call the group. For example, the Candidate Phyla Radiation includes the Patescibacteria group which includes Microgenomates group which includes over 11 bacterial phyla.
Candidate phyla radiation (CPR)
The CPR is a descriptive term referring to a massive monophyletic radiation of candidate phyla that exists within the Bacterial domain.[63] It includes two main clades, the Microgenomates and Parcubacteria groups, each containing the eponymous superphyla and a few other phyla.
Patescibacteria
The superphylum Patescibacteria was originally proposed to encompass the phyla Microgenomates (OP11), Parcubacteria (OD1), and Gracilibacteria (GNO2 / BD1-5).[24] More recent phylogenetic analyses show that the last common ancestor of these taxa is the same node as that of CPR.[64]
Sphingobacteria
The Sphingobacteria (FCB group) includes Bacteroidetes, Calditrichaeota, Chlorobi, candidate phylum Cloacimonetes, Fibrobacteres, Gemmatimonadates, candidate phylum Ignavibacteriae, candidate phylum Latescibacteria, candidate phylum Marinimicrobia, and candidate phylum Zixibacteria.[24][65]
Microgenomates
Microgenomates was originally thought to be a single phylum although evidence suggests it actually encompasses over 11 bacterial phyla,[16][4] including Curtisbacteria, Daviesbacteria, Levybacteria, Gottesmanbacteria, Woesebacteria, Amesbacteria, Shapirobacteria, Roizmanbacteria, Beckwithbacteria, Collierbacteria, Pacebacteria.
Parcubacteria
Parcubacteria was originally described as a single phylum using fewer than 100 16S rRNA sequences. With a greater diversity of 16S rRNA sequences from uncultured organisms now available, it is estimated it may consist of up to 28 bacterial phyla.[2] In line with this, over 14 phyla have now been described within the Parcubacteria group,[16][4] including Kaiserbacteria, Adlerbacteria, Campbellbacteria, Nomurabacteria, Giovannonibacteria, Wolfebacteria, Jorgensenbacteria, Yanofskybacteria, Azambacteria, Moranbacteria, Uhrbacteria, and Magasanikbacteria.
Proteobacteria
It has been proposed that some classes of the phylum Proteobacteria may be phyla in their own right, which would make Proteobacteria a superphylum.[66] For example, the Deltaproteobacteria group does not consistently form a monophyletic lineage with the other Proteobacteria classes.[67]
Planctobacteria
The Planctobacteria (PVC group) includes Chlamydiae, Lentisphaerae, candidate phylum Omnitrophica, Planctomycetes, candidate phylum Poribacteria, and Verrucomicrobia.[24][65]
Terrabacteria
The proposed superphylum, Terrabacteria,[68] includes Actinobacteria, Cyanobacteria, Deinococcus–Thermus, Chloroflexi, Firmicutes, and candidate phylum OP10.[68][69][24][65]
Cryptic superphyla
Several candidate phyla (Microgenomates, Omnitrophica, Parcubacteria, and Saccharibacteria) and several accepted phyla (Elusimicrobia, Caldiserica, and Armatimonadetes) have been suggested to actually be superphyla that were incorrectly described as phyla because rules for defining a bacterial phylum are lacking or due to a lack of sequence diversity in databases when the phylum was first establish.[2] For example, it is suggested that candidate phylum Parcubacteria is actually a superphylum that encompasses 28 subordinate phyla and that phylum Elusimicrobia is actually a superphylum that encompasses 7 subordinate phyla.[66]
Historical perspective
Given the rich history of the field of bacterial taxonomy and the rapidity of changes therein in modern times, it is often useful to have a historical perspective on how the field has progressed in order to understand references to antiquated definitions or concepts.
When bacterial nomenclature was controlled under the Botanical Code, the term division was used, but now that bacterial nomenclature (with the exception of cyanobacteria) is controlled under the Bacteriological Code, the term phylum is preferred.
In 1987, Carl Woese, regarded as the forerunner of the molecular phylogeny revolution, divided Eubacteria into 11 divisions based on 16S ribosomal RNA (SSU) sequences, listed below.[71][72]
- Purple Bacteria and their relatives (later renamed Proteobacteria[73])
- alpha subdivision (purple non-sulfur bacteria, rhizobacteria, Agrobacterium, Rickettsiae, Nitrobacter)
- beta subdivision (Rhodocyclus, (some) Thiobacillus, Alcaligenes, Spirillum, Nitrosovibrio)
- gamma subdivision (enterics, fluorescent pseudomonads, purple sulfur bacteria, Legionella, (some) Beggiatoa)
- delta subdivision (Sulfur and sulfate reducers (Desulfovibrio), Myxobacteria, Bdellovibrio)
- Gram-positive Eubacteria[Note 1]
- High-G+C species (later renamed Actinobacteria[77]) (Actinomyces, Streptomyces, Arthrobacter, Micrococcus, Bifidobacterium)
- Low-G+C species (later renamed Firmicutes[77]) (Clostridium, Peptococcus, Bacillus, Mycoplasma)
- Photosynthetic species (Heliobacteria)
- Species with Gram-negative walls (Megasphaera, Sporomusa)
- Cyanobacteria and chloroplasts (Aphanocapsa, Oscillatoria, Nostoc, Synechococcus, Gloeobacter, Prochloron)
- Spirochetes and relatives
- Spirochetes (Spirochaeta, Treponema, Borrelia)
- Leptospiras (Leptospira, Leptonema)
- Green sulfur bacteria (Chlorobium, Chloroherpeton)
- Bacteroides, Flavobacteria and relatives (later renamed Bacteroidetes
- Bacteroides (Bacteroides, Fusobacterium)
- Flavobacterium group (Flavobacterium, Cytophaga, Saprospira, Flexibacter)
- Planctomyces and relatives (later renamed Planctomycetes)
- Chlamydiae (Chlamydia psittaci, Chlamydia trachomatis)
- Radioresistant micrococci and relatives (now commonly referred to as Deinococcus–Thermus[78] or Thermi)[Note 3]
- Deinococcus group (Deinococcus radiodurans)
- Thermophiles (Thermus aquaticus)
- Green non-sulfur bacteria and relatives (later renamed Chloroflexi[82])
- Chloroflexus group (Chloroflexus, Herpetosiphon)
- Thermomicrobium group (Thermomicrobium roseum)
- Thermotogae (Thermotoga maritima)
Traditionally, phylogeny was inferred and taxonomy established based on studies of morphology. The advent of molecular phylogenetics has allowed for improved elucidation of the evolutionary relationship of species by analyzing their DNA and protein sequences, for example their ribosomal DNA.[83] The lack of easily accessible morphological features, such as those present in animals and plants, hampered early efforts of classification and resulted in erroneous, distorted and confused classification, an example of which, noted Carl Woese, is Pseudomonas whose etymology ironically matched its taxonomy, namely "false unit".[71] Many bacterial taxa were re-classified or re-defined using molecular phylogenetics.
The advent of molecular sequencing technologies has allowed for the recovery of genomes directly from environmental samples (i.e. bypassing culturing), leading to rapid expansion of our knowledge of the diversity of bacterial phyla. These techniques are genome-resolved metagenomics and single-cell genomics.
See also
Footnotes
- Until recently, it was believed than only Firmicutes and Actinobacteria were Gram-positive. However, the candidate phylum TM7 may also be Gram positive.[74] Chloroflexi however possess a single bilayer, but stain negative (with some exceptions[75]).[76]
- Pasteuria is now assigned to phylum Bacilli, not to phylum Planctomycetes.
- It has been proposed to call the clade Xenobacteria[79] or Hadobacteria[80] (the latter is considered an illegitimate name[81]).
References
- Hug, Laura A.; Baker, Brett J.; Anantharaman, Karthik; Brown, Christopher T.; Probst, Alexander J.; Castelle, Cindy J.; Butterfield, Cristina N.; Hernsdorf, Alex W.; Amano, Yuki; Ise, Kotaro; Suzuki, Yohey (11 April 2016). "A new view of the tree of life". Nature Microbiology. 1 (5): 16048. doi:10.1038/nmicrobiol.2016.48. ISSN 2058-5276. PMID 27572647.
- Yarza, Pablo; Yilmaz, Pelin; Pruesse, Elmar; Glöckner, Frank Oliver; Ludwig, Wolfgang; Schleifer, Karl-Heinz; Whitman, William B.; Euzéby, Jean; Amann, Rudolf; Rosselló-Móra, Ramon (September 2014). "Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences". Nature Reviews Microbiology. 12 (9): 635–645. doi:10.1038/nrmicro3330. ISSN 1740-1534. PMID 25118885. S2CID 21895693.
- Bacterial phyla entry in LPSN; Euzéby, J.P. (1997). "List of Bacterial Names with Standing in Nomenclature: a folder available on the Internet". International Journal of Systematic and Evolutionary Microbiology. 47 (2): 590–2. doi:10.1099/00207713-47-2-590. PMID 9103655.
- Anantharaman, Karthik; Brown, Christopher T.; Hug, Laura A.; Sharon, Itai; Castelle, Cindy J.; Probst, Alexander J.; Thomas, Brian C.; Singh, Andrea; Wilkins, Michael J.; Karaoz, Ulas; Brodie, Eoin L. (24 October 2016). "Thousands of microbial genomes shed light on interconnected biogeochemical processes in an aquifer system". Nature Communications. 7 (1): 13219. Bibcode:2016NatCo...713219A. doi:10.1038/ncomms13219. ISSN 2041-1723. PMC 5079060. PMID 27774985.
- Parks, Donovan H.; Rinke, Christian; Chuvochina, Maria; Chaumeil, Pierre-Alain; Woodcroft, Ben J.; Evans, Paul N.; Hugenholtz, Philip; Tyson, Gene W. (November 2017). "Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life". Nature Microbiology. 2 (11): 1533–1542. doi:10.1038/s41564-017-0012-7. ISSN 2058-5276. PMID 28894102.
- Dudek, Natasha K.; Sun, Christine L.; Burstein, David; Kantor, Rose S.; Aliaga Goltsman, Daniela S.; Bik, Elisabeth M.; Thomas, Brian C.; Banfield, Jillian F.; Relman, David A. (18 December 2017). "Novel Microbial Diversity and Functional Potential in the Marine Mammal Oral Microbiome". Current Biology. 27 (24): 3752–3762.e6. doi:10.1016/j.cub.2017.10.040. ISSN 1879-0445. PMID 29153320.
- "ARB-Silva: comprehensive ribosomal RNA database". The ARB development Team. Retrieved 2 January 2016.
- Alexander L. Jaffe, Cindy J. Castelle, Paula B. Matheus Carnevali, Simonetta Gribaldo, Jillian F. Banfield: The rise of diversity in metabolic platforms across the Candidate Phyla Radiation. In: BMC Biology Vol. 18, Nr. 69; June 2020); doi:10.1186/s12915-020-00804-5
- Tahon, Guillaume; Tytgat, Bjorn; Lebbe, Liesbeth; Carlier, Aurélien; Willems, Anne (1 July 2018). "Abditibacterium utsteinense sp. nov., the first cultivated member of candidate phylum FBP, isolated from ice-free Antarctic soil samples". Systematic and Applied Microbiology. 41 (4): 279–290. doi:10.1016/j.syapm.2018.01.009. ISSN 0723-2020. PMID 29475572.
- Harris, J. Kirk; Kelley, Scott T.; Pace, Norman R. (February 2004). "New Perspective on Uncultured Bacterial Phylogenetic Division OP11". Applied and Environmental Microbiology. 70 (2): 845–849. doi:10.1128/AEM.70.2.845-849.2004. ISSN 0099-2240. PMC 348892. PMID 14766563.
- Rappé, Michael S.; Giovannoni, Stephen J. (2003). "The Uncultured Microbial Majority". Annual Review of Microbiology. 57: 369–94. doi:10.1146/annurev.micro.57.030502.090759. PMID 14527284.
- Kenly A. Hiller, Kenneth H. Foreman, David Weisman, Jennifer L. Bowen: Permeable Reactive Barriers Designed To Mitigate Eutrophication Alter Bacterial Community Composition and Aquifer Redox Conditions. In: Appl Environ Microbiol v.81(20); 2015 Oct; pp.7114–7124. doi:10.1128/AEM.01986-15. PMC 4579450. PMID 26231655.
- Hugenholtz P; et al. (1998). "Novel division level bacterial diversity in a Yellowstone hot spring". Journal of Bacteriology. 180 (2): 366–76. doi:10.1128/JB.180.2.366-376.1998. PMC 106892. PMID 9440526.
- Thrash, J. Cameron; Coates, John D. (2010), "Phylum XVII. Acidobacteria phyl. nov.", Bergey’s Manual® of Systematic Bacteriology, Springer New York, pp. 725–735, doi:10.1007/978-0-387-68572-4_6, ISBN 978-0-387-95042-6
- Goodfellow, Michael (2012). "Phylum XXVI. Actinobacteria phyl. Nov.". Bergey's Manual® of Systematic Bacteriology. Springer New York. pp. 33–2028. doi:10.1007/978-0-387-68233-4_3. ISBN 978-0-387-95043-3.
- Christopher T. Brown, Laura A. Hug, Brian C. Thomas et al.; et al. (2015). "Unusual biology across a group comprising more than 15% of domain Bacteria". Nature. 523 (7559): 208–11. Bibcode:2015Natur.523..208B. doi:10.1038/nature14486. OSTI 1512215. PMID 26083755. S2CID 4397558.CS1 maint: multiple names: authors list (link)
- Tamaki, Hideyuki; Tanaka, Yasuhiro; Matsuzawa, Hiroaki; Muramatsu, Mizuho; Meng, Xian-Ying; Hanada, Satoshi; Mori, Kazuhiro; Kamagata, Yoichi (June 2011). "Armatimonas rosea gen. nov., sp. nov., of a novel bacterial phylum, Armatimonadetes phyl. nov., formally called the candidate phylum OP10". International Journal of Systematic and Evolutionary Microbiology. 61 (Pt 6): 1442–1447. doi:10.1099/ijs.0.025643-0. ISSN 1466-5034. PMID 20622056.
- Hahnke, Richard L.; Meier-Kolthoff, Jan P.; García-López, Marina; Mukherjee, Supratim; Huntemann, Marcel; Ivanova, Natalia N.; Woyke, Tanja; Kyrpides, Nikos C.; Klenk, Hans-Peter; Göker, Markus (2016). "Genome-Based Taxonomic Classification of Bacteroidetes". Frontiers in Microbiology. 7: 2003. doi:10.3389/fmicb.2016.02003. ISSN 1664-302X. PMC 5167729. PMID 28066339.
- Wrighton, Kelly C.; Castelle, Cindy J.; Wilkins, Michael J.; Hug, Laura A.; Sharon, Itai; Thomas, Brian C.; Handley, Kim M.; Mullin, Sean W.; Nicora, Carrie D.; Singh, Andrea; Lipton, Mary S. (July 2014). "Metabolic interdependencies between phylogenetically novel fermenters and respiratory organisms in an unconfined aquifer". The ISME Journal. 8 (7): 1452–1463. doi:10.1038/ismej.2013.249. ISSN 1751-7370. PMC 4069391. PMID 24621521.
- Robert E. Danczak, M. D. Johnston, C. Kenah, M. Slattery, K. C. Wrighton, M. J. Wilkins (September 2017). "Members of the candidate phyla radiation are functionally differentiated by carbon- and nitrogen-cycling capabilities". Microbiome. 5 (1): 112. doi:10.1186/s40168-017-0331-1. PMC 5581439. PMID 28865481.CS1 maint: multiple names: authors list (link)
- Dunfield, Peter F.; Tamas, Ivica; Lee, Kevin C.; Morgan, Xochitl C.; McDonald, Ian R.; Stott, Matthew B. (2012). "Electing a candidate: a speculative history of the bacterial phylum OP10". Environmental Microbiology. 14 (12): 3069–3080. doi:10.1111/j.1462-2920.2012.02742.x. ISSN 1462-2920. PMID 22497633.
- Mori, K.; Yamaguchi, K.; Sakiyama, Y.; Urabe, T.; Suzuki, K.-i. (23 July 2009). "Caldisericum exile gen. nov., sp. nov., an anaerobic, thermophilic, filamentous bacterium of a novel bacterial phylum, Caldiserica phyl. nov., originally called the candidate phylum OP5, and description of Caldisericaceae fam. nov., Caldisericales ord. nov. and Caldisericia classis nov". International Journal of Systematic and Evolutionary Microbiology. 59 (11): 2894–2898. doi:10.1099/ijs.0.010033-0. ISSN 1466-5026. PMID 19628600.
- Kublanov, Ilya V.; Sigalova, Olga M.; Gavrilov, Sergey N.; Lebedinsky, Alexander V.; Rinke, Christian; Kovaleva, Olga; Chernyh, Nikolai A.; Ivanova, Natalia; Daum, Chris; Reddy, T.B.K.; Klenk, Hans-Peter (20 February 2017). "Genomic Analysis of Caldithrix abyssi, the Thermophilic Anaerobic Bacterium of the Novel Bacterial Phylum Calditrichaeota". Frontiers in Microbiology. 8: 195. doi:10.3389/fmicb.2017.00195. ISSN 1664-302X. PMC 5317091. PMID 28265262.
- Rinke C; et al. (2013). "Insights into the phylogeny and coding potential of microbial dark matter". Nature. 499 (7459): 431–7. Bibcode:2013Natur.499..431R. doi:10.1038/nature12352. PMID 23851394.
- Boone, David R.; Castenholz, Richard W.; Garrity, George M., eds. (2001). Bergey's Manual® of Systematic Bacteriology. doi:10.1007/978-0-387-21609-6. ISBN 978-1-4419-3159-7. S2CID 41426624.
- Chouari, Rakia; Le Paslier, Denis; Dauga, Catherine; Daegelen, Patrick; Weissenbach, Jean; Sghir, Abdelghani (April 2005). "Novel Major Bacterial Candidate Division within a Municipal Anaerobic Sludge Digester". Applied and Environmental Microbiology. 71 (4): 2145–2153. doi:10.1128/aem.71.4.2145-2153.2005. ISSN 0099-2240. PMC 1082523. PMID 15812049.
- Hug, Laura A.; Thomas, Brian C.; Sharon, Itai; Brown, Christopher T.; Sharma, Ritin; Hettich, Robert L.; Wilkins, Michael J.; Williams, Kenneth H.; Singh, Andrea; Banfield, Jillian F. (2016). "Critical biogeochemical functions in the subsurface are associated with bacteria from new phyla and little studied lineages". Environmental Microbiology. 18 (1): 159–173. doi:10.1111/1462-2920.12930. ISSN 1462-2920. OSTI 1328276. PMID 26033198.
- Rheims, H; Rainey, F A; Stackebrandt, E (September 1996). "A molecular approach to search for diversity among bacteria in the environment". Journal of Industrial Microbiology & Biotechnology. 17 (3–4): 159–169. doi:10.1007/bf01574689. ISSN 0169-4146. S2CID 31868442.
- Patel, Bharat K. C. (2010). "Phylum XX. Dictyoglomi phyl. Nov.". Bergey's Manual® of Systematic Bacteriology. Springer New York. pp. 775–780. doi:10.1007/978-0-387-68572-4_9. ISBN 978-0-387-95042-6.
- Ji, Mukan; Greening, Chris; Vanwonterghem, Inka; Carere, Carlo R.; Bay, Sean K.; Steen, Jason A.; Montgomery, Kate; Lines, Thomas; Beardall, John; van Dorst, Josie; Snape, Ian (December 2017). "Atmospheric trace gases support primary production in Antarctic desert surface soil". Nature. 552 (7685): 400–403. Bibcode:2017Natur.552..400J. doi:10.1038/nature25014. ISSN 1476-4687. PMID 29211716.
- Youssef, Noha H.; Farag, Ibrahim F.; Hahn, C. Ryan; Premathilake, Hasitha; Fry, Emily; Hart, Matthew; Huffaker, Krystal; Bird, Edward; Hambright, Jimmre; Hoff, Wouter D.; Elshahed, Mostafa S. (1 January 2019). "Candidatus Krumholzibacterium zodletonense gen. nov., sp nov, the first representative of the candidate phylum Krumholzibacteriota phyl. nov. recovered from an anoxic sulfidic spring using genome resolved metagenomics". Systematic and Applied Microbiology. Taxonomy of uncultivated Bacteria and Archaea. 42 (1): 85–93. doi:10.1016/j.syapm.2018.11.002. ISSN 0723-2020. PMID 30477901.
- Herlemann, D. P. R.; Geissinger, O.; Ikeda-Ohtsubo, W.; Kunin, V.; Sun, H.; Lapidus, A.; Hugenholtz, P.; Brune, A. (1 May 2009). "Genomic Analysis of "Elusimicrobium minutum," the First Cultivated Representative of the Phylum "Elusimicrobia" (Formerly Termite Group 1)". Applied and Environmental Microbiology. 75 (9): 2841–2849. doi:10.1128/AEM.02698-08. ISSN 0099-2240. PMC 2681670. PMID 19270133.
- Nogales, Balbina; Moore, Edward R. B.; Llobet-Brossa, Enrique; Rossello-Mora, Ramon; Amann, Rudolf; Timmis, Kenneth N. (1 April 2001). "Combined Use of 16S Ribosomal DNA and 16S rRNA To Study the Bacterial Community of Polychlorinated Biphenyl-Polluted Soil". Applied and Environmental Microbiology. 67 (4): 1874–1884. doi:10.1128/AEM.67.4.1874-1884.2001. ISSN 0099-2240. PMC 92809. PMID 11282645.
- Ward, Lewis M.; Cardona, Tanai; Holland-Moritz, Hannah (29 January 2019). "Evolutionary Implications of Anoxygenic Phototrophy in the Bacterial Phylum Candidatus Palusbacterota (WPS-2)". doi:10.1101/534180. S2CID 92796436. Cite journal requires
|journal=(help) - Knittel, Katrin; Boetius, Antje; Lemke, Andreas; Eilers, Heike; Lochte, Karin; Pfannkuche, Olaf; Linke, Peter; Amann, Rudolf (July 2003). "Activity, Distribution, and Diversity of Sulfate Reducers and Other Bacteria in Sediments above Gas Hydrate (Cascadia Margin, Oregon)". Geomicrobiology Journal. 20 (4): 269–294. doi:10.1080/01490450303896. ISSN 0149-0451. S2CID 140639772.
- Zhang, Hui; Sekiguchi, Yuji; Hanada, Satoshi; Hugenholtz, Philip; Kim, Hongik; Kamagata, Yoichi; Nakamura, Kazunori (2003). "Gemmatimonas aurantiaca gen. nov., sp. nov., a Gram-negative, aerobic, polyphosphate-accumulating micro-organism, the first cultured representative of the new bacterial phylum Gemmatimonadetes phyl. nov". International Journal of Systematic and Evolutionary Microbiology. 53 (4): 1155–1163. doi:10.1099/ijs.0.02520-0. ISSN 1466-5026. PMID 12892144.
- Ley, Ruth E.; Harris, J. Kirk; Wilcox, Joshua; Spear, John R.; Miller, Scott R.; Bebout, Brad M.; Maresca, Julia A.; Bryant, Donald A.; Sogin, Mitchell L.; Pace, Norman R. (1 May 2006). "Unexpected Diversity and Complexity of the Guerrero Negro Hypersaline Microbial Mat". Applied and Environmental Microbiology. 72 (5): 3685–3695. doi:10.1128/AEM.72.5.3685-3695.2006. ISSN 0099-2240. PMC 1472358. PMID 16672518.
- Guermazi, Sonda; Daegelen, Patrick; Dauga, Catherine; Rivière, Delphine; Bouchez, Théodore; Godon, Jean Jacques; Gyapay, Gábor; Sghir, Abdelghani; Pelletier, Eric; Weissenbach, Jean; Le Paslier, Denis (August 2008). "Discovery and characterization of a new bacterial candidate division by an anaerobic sludge digester metagenomic approach". Environmental Microbiology. 10 (8): 2111–2123. doi:10.1111/j.1462-2920.2008.01632.x. ISSN 1462-2912. PMC 2702496. PMID 18459975.
- Eloe-Fadrosh, Emiley A.; Paez-Espino, David; Jarett, Jessica; Dunfield, Peter F.; Hedlund, Brian P.; Dekas, Anne E.; Grasby, Stephen E.; Brady, Allyson L.; Dong, Hailiang; Briggs, Brandon R.; Li, Wen-Jun (27 January 2016). "Global metagenomic survey reveals a new bacterial candidate phylum in geothermal springs". Nature Communications. 7 (1): 10476. Bibcode:2016NatCo...710476E. doi:10.1038/ncomms10476. ISSN 2041-1723. PMC 4737851. PMID 26814032.
- Youssef, Noha H.; Farag, Ibrahim F.; Hahn, C. Ryan; Jarett, Jessica; Becraft, Eric; Eloe-Fadrosh, Emiley; Lightfoot, Jorge; Bourgeois, Austin; Cole, Tanner; Ferrante, Stephanie; Truelock, Mandy (15 May 2019). "Genomic Characterization of Candidate Division LCP-89 Reveals an Atypical Cell Wall Structure, Microcompartment Production, and Dual Respiratory and Fermentative Capacities". Applied and Environmental Microbiology. 85 (10). doi:10.1128/AEM.00110-19. ISSN 0099-2240. PMC 6498177. PMID 30902854.
- NCBI: Candidatus Magasanikbacteria (phylum)
- Di Rienzi, Sara C; Sharon, Itai; Wrighton, Kelly C; Koren, Omry; Hug, Laura A; Thomas, Brian C; Goodrich, Julia K; Bell, Jordana T; Spector, Timothy D; Banfield, Jillian F; Ley, Ruth E (1 October 2013). "The human gut and groundwater harbor non-photosynthetic bacteria belonging to a new candidate phylum sibling to Cyanobacteria". eLife. 2: e01102. doi:10.7554/eLife.01102. ISSN 2050-084X. PMC 3787301. PMID 24137540.
- Hugenholtz, Philip; Goebel, Brett M.; Pace, Norman R. (15 December 1998). "Impact of Culture-Independent Studies on the Emerging Phylogenetic View of Bacterial Diversity". Journal of Bacteriology. 180 (24): 4765–74. doi:10.1128/jb.180.24.6793-6793.1998. ISSN 1098-5530. PMC 107498. PMID 9733676.
- Sekiguchi, Yuji; Ohashi, Akiko; Parks, Donovan H.; Yamauchi, Toshihiro; Tyson, Gene W.; Hugenholtz, Philip (27 January 2015). "First genomic insights into members of a candidate bacterial phylum responsible for wastewater bulking". PeerJ. 3: e740. doi:10.7717/peerj.740. ISSN 2167-8359. PMC 4312070. PMID 25650158.
- Holmes, Andrew J.; Tujula, Niina A.; Holley, Marita; Contos, Annalisa; James, Julia M.; Rogers, Peter; Gillings, Michael R. (2001). "Phylogenetic structure of unusual aquatic microbial formations in Nullarbor caves, Australia". Environmental Microbiology. 3 (4): 256–264. doi:10.1046/j.1462-2920.2001.00187.x. ISSN 1462-2920. PMID 11359511.
- Luecker, Sebastian; Nowka, Boris; Rattei, Thomas; Spieck, Eva; Daims, Holger (2013). "The Genome of Nitrospina gracilis Illuminates the Metabolism and Evolution of the Major Marine Nitrite Oxidizer". Frontiers in Microbiology. 4: 27. doi:10.3389/fmicb.2013.00027. ISSN 1664-302X. PMC 3578206. PMID 23439773.
- LPSN: Phylum "Candidatus Parcunitrobacteria"
- Cindy J. Castelle, Christopher T. Brown, Brian C. Thomas, Kenneth H. Williams, Jillian F. Banfield: Unusual respiratory capacity and nitrogen metabolism in a Parcubacterium (OD1) of the Candidate Phyla Radiation. In: Sci Rep 7, 40101; Jan 9, 2017; doi:10.1038/srep40101
- Astudillo‐García, Carmen; Slaby, Beate M.; Waite, David W.; Bayer, Kristina; Hentschel, Ute; Taylor, Michael W. (2018). "Phylogeny and genomics of SAUL, an enigmatic bacterial lineage frequently associated with marine sponges" (PDF). Environmental Microbiology. 20 (2): 561–576. doi:10.1111/1462-2920.13965. ISSN 1462-2920. PMID 29098761. S2CID 23892350.
- Wrighton, K. C.; Thomas, B. C.; Sharon, I.; Miller, C. S.; Castelle, C. J.; VerBerkmoes, N. C.; Wilkins, M. J.; Hettich, R. L.; Lipton, M. S.; Williams, K. H.; Long, P. E. (27 September 2012). "Fermentation, Hydrogen, and Sulfur Metabolism in Multiple Uncultivated Bacterial Phyla". Science. 337 (6102): 1661–1665. Bibcode:2012Sci...337.1661W. doi:10.1126/science.1224041. ISSN 0036-8075. PMID 23019650. S2CID 10362580.
- NCBI: Candidatus Peregrinibacteria (phylum)
- UniProt: Taxonomy - Candidatus Peregrinibacteria (PHYLUM)
- Karthik Anantharaman, Christopher T. Brown, David Burstein, Cindy Castelle: Analysis of five complete genome sequences for members of the class Peribacteria in the recently recognized Peregrinibacteria bacterial phylum. In: PeerJ 4(8):e1607; Jan 2016; doi:10.7717/peerj.1607
- Fieseler, Lars; Horn, Matthias; Wagner, Michael; Hentschel, Ute (June 2004). "Discovery of the Novel Candidate Phylum "Poribacteria" in Marine Sponges". Applied and Environmental Microbiology. 70 (6): 3724–3732. doi:10.1128/AEM.70.6.3724-3732.2004. ISSN 0099-2240. PMC 427773. PMID 15184179.
- Wiegand, Sandra; Jogler, Mareike; Kohn, Timo; Awal, Ram Prasad; Oberbeckmann, Sonja; Kesy, Katharina; Jeske, Olga; Schumann, Peter; Peeters, Stijn H. (24 October 2019). "The novel shapeshifting bacterial phylum Saltatorellota". doi:10.1101/817700. S2CID 208566371. Cite journal requires
|journal=(help) - Derakshani, Manigee; Lukow, Thomas; Liesack, Werner (1 February 2001). "Novel Bacterial Lineages at the (Sub)Division Level as Detected by Signature Nucleotide-Targeted Recovery of 16S rRNA Genes from Bulk Soil and Rice Roots of Flooded Rice Microcosms". Applied and Environmental Microbiology. 67 (2): 623–631. doi:10.1128/aem.67.2.623-631.2001. ISSN 1098-5336. PMC 92629. PMID 11157225.
- Kadnikov, Vitaly V.; Mardanov, Andrey V.; Beletsky, Alexey V.; Rakitin, Andrey L.; Frank, Yulia A.; Karnachuk, Olga V.; Ravin, Nikolai V. (January 2019). "Phylogeny and physiology of candidate phylum BRC1 inferred from the first complete metagenome-assembled genome obtained from deep subsurface aquifer". Systematic and Applied Microbiology. 42 (1): 67–76. doi:10.1016/j.syapm.2018.08.013. ISSN 1618-0984. PMID 30201528.
- Baker, Brett J.; Lazar, Cassandre Sara; Teske, Andreas P.; Dick, Gregory J. (13 April 2015). "Genomic resolution of linkages in carbon, nitrogen, and sulfur cycling among widespread estuary sediment bacteria". Microbiome. 3 (1): 14. doi:10.1186/s40168-015-0077-6. ISSN 2049-2618. PMC 4411801. PMID 25922666.
- Wilson, Micheal C.; Mori, Tetsushi; Rückert, Christian; Uria, Agustinus R.; Helf, Maximilian J.; Takada, Kentaro; Gernert, Christine; Steffens, Ursula A. E.; Heycke, Nina; Schmitt, Susanne; Rinke, Christian (February 2014). "An environmental bacterial taxon with a large and distinct metabolic repertoire". Nature. 506 (7486): 58–62. Bibcode:2014Natur.506...58W. doi:10.1038/nature12959. ISSN 1476-4687. PMID 24476823.
- Reysenbach, Anna-Louise; Huber, Robert; Stetter, Karl O.; Davey, Mary Ellen; MacGregor, Barbara J.; Stahl, David A. (2001), "Phylum BII. Thermotogae phy. nov.", Bergey’s Manual® of Systematic Bacteriology, Springer New York, pp. 369–387, doi:10.1007/978-0-387-21609-6_19, ISBN 978-1-4419-3159-7
- Probst, AJ; Castelle, CJ; Singh, A; Brown, CT; Anantharaman, K; Sharon, I; Hug, LA; Burstein, D; Emerson, JB; Thomas, BC; Banfield, JF (February 2017). "Genomic resolution of a cold subsurface aquifer community provides metabolic insights for novel microbes adapted to high CO2 concentrations". Environmental Microbiology. 19 (2): 459–474. doi:10.1111/1462-2920.13362. PMID 27112493. S2CID 21126011.
- Castelle, Cindy J.; Hug, Laura A.; Wrighton, Kelly C.; Thomas, Brian C.; Williams, Kenneth H.; Wu, Dongying; Tringe, Susannah G.; Singer, Steven W.; Eisen, Jonathan A.; Banfield, Jillian F. (27 August 2013). "Extraordinary phylogenetic diversity and metabolic versatility in aquifer sediment". Nature Communications. 4 (1): 2120. Bibcode:2013NatCo...4.2120C. doi:10.1038/ncomms3120. ISSN 2041-1723. PMC 3903129. PMID 23979677.
- Castelle CJ, Banfield JF (March 2018). "Major New Microbial Groups Expand Diversity and Alter our Understanding of the Tree of Life". Cell. 172 (6): 1181–1197. doi:10.1016/j.cell.2018.02.016. PMID 29522741.
- Castelle, Cindy J.; Banfield, Jillian F. (8 March 2018). "Major New Microbial Groups Expand Diversity and Alter our Understanding of the Tree of Life". Cell. 172 (6): 1181–1197. doi:10.1016/j.cell.2018.02.016. ISSN 0092-8674. PMID 29522741.
- Sekiguchi Y; et al. (2015). "First genomic insights into members of a candidate bacterial phylum responsible for wastewater bulking". PeerJ. 3: e740. doi:10.7717/peerj.740. PMC 4312070. PMID 25650158.
- Yarza P; et al. (2014). "Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences". Nature Reviews Microbiology. 12 (9): 635–645. doi:10.1038/nrmicro3330. hdl:10261/123763. PMID 25118885. S2CID 21895693.
- Hug LA; et al. (2016). "A new view of the tree of life". Nature Microbiology. Article 16048 (5): 16048. doi:10.1038/nmicrobiol.2016.48. PMID 27572647.
- Battistuzzi FU, Feijao A, Hedges SB (November 2004). "A genomic timescale of prokaryote evolution: insights into the origin of methanogenesis, phototrophy, and the colonization of land". BMC Evolutionary Biology. 4: 44. doi:10.1186/1471-2148-4-44. PMC 533871. PMID 15535883.
- Battistuzzi, F. U.; Hedges, S. B. (6 November 2008). "A Major Clade of Prokaryotes with Ancient Adaptations to Life on Land". Molecular Biology and Evolution. 26 (2): 335–343. doi:10.1093/molbev/msn247. PMID 18988685.
- Schluenzen F; et al. (2000). "Structure of functionally activated small ribosomal subunit at 3.3 angstroms resolution". Cell. 102 (5): 615–23. doi:10.1016/S0092-8674(00)00084-2. PMID 11007480. S2CID 1024446.
- Woese, CR (1987). "Bacterial evolution". Microbiological Reviews. 51 (2): 221–71. doi:10.1128/MMBR.51.2.221-271.1987. PMC 373105. PMID 2439888.
- Holland L (22 May 1990). "Carl Woese in forefront of bacterial evolution revolution". The Scientist. 3 (10).
- Stackebrandt; et al. (1988). "Proteobacteria classis nov., a name for the phylogenetic taxon that includes the "purple bacteria and their relatives"". Int. J. Syst. Bacteriol. 38 (3): 321–325. doi:10.1099/00207713-38-3-321.
- Hugenholtz, P.; Tyson, G. W.; Webb, R. I.; Wagner, A. M.; Blackall, L. L. (2001). "Investigation of Candidate Division TM7, a Recently Recognized Major Lineage of the Domain Bacteria with No Known Pure-Culture Representatives". Applied and Environmental Microbiology. 67 (1): 411–9. doi:10.1128/AEM.67.1.411-419.2001. PMC 92593. PMID 11133473.
- Yabe, S.; Aiba, Y.; Sakai, Y.; Hazaka, M.; Yokota, A. (2010). "Thermogemmatispora onikobensis gen. nov., sp. nov. and Thermogemmatispora foliorum sp. nov., isolated from fallen leaves on geothermal soils, and description of Thermogemmatisporaceae fam. nov. and Thermogemmatisporales ord. nov. within the class Ktedonobacteria". International Journal of Systematic and Evolutionary Microbiology. 61 (4): 903–910. doi:10.1099/ijs.0.024877-0. PMID 20495028.
- Sutcliffe, I. C. (2011). "Cell envelope architecture in the Chloroflexi: A shifting frontline in a phylogenetic turf war". Environmental Microbiology. 13 (2): 279–282. doi:10.1111/j.1462-2920.2010.02339.x. PMID 20860732.
- Stackebrandt, E.; Rainey, F. A.; Ward-Rainey, N. L. (1997). "Proposal for a New Hierarchic Classification System, Actinobacteria classis nov". International Journal of Systematic Bacteriology. 47 (2): 479–491. doi:10.1099/00207713-47-2-479.
- J.P. Euzéby. "List of Prokaryotic names with Standing in Nomenclature: classification of Deinococcus–Thermus". Archived from the original on 27 January 2013. Retrieved 30 December 2010.
- Bergey's Manual of Systematic Bacteriology 1st Ed.
- Cavalier-Smith, T (2002). "The neomuran origin of Archaebacteria, the negibacterial root of the universal tree and bacterial megaclassification". International Journal of Systematic and Evolutionary Microbiology. 52 (Pt 1): 7–76. doi:10.1099/00207713-52-1-7. PMID 11837318.
- "List of Prokaryotic names with Standing in Nomenclature—Class Hadobacteria". LPSN. Archived from the original on 19 April 2012. Retrieved 30 December 2010. Euzéby, J.P. (1997). "List of Bacterial Names with Standing in Nomenclature: a folder available on the Internet". Int J Syst Bacteriol. 47 (2): 590–2. doi:10.1099/00207713-47-2-590. ISSN 0020-7713. PMID 9103655.
- Boone DR; Castenholz RW (18 May 2001) [1984 (Williams & Wilkins)]. Garrity GM (ed.). The Archaea and the Deeply Branching and Phototrophic Bacteria. Bergey's Manual of Systematic Bacteriology. 1 (2nd ed.). New York: Springer. pp. 721. ISBN 978-0-387-98771-2. British Library no. GBA561951.
- Olsen GJ, Woese CR, Overbeek R (1994). "The winds of (evolutionary) change: breathing new life into microbiology". Journal of Bacteriology. 176 (1): 1–6. doi:10.2172/205047. PMC 205007. PMID 8282683.