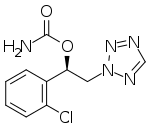
Cenobamate
Cenobamate, sold under the brand name Xcopri, is a medication used for the treatment of partial-onset seizures in adults.[3][4]
 | |
| Clinical data | |
|---|---|
| Trade names | Xcopri |
| Other names | YKP3089 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620021 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| PubChem SID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C10H10ClN5O2 |
| Molar mass | 267.67 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
It was approved for medical use in the United States in November 2019[3][4][5] and placed in Schedule V in March 2020.[6]
Pharmacology
Pharmacodynamics
Cenobamate is a voltage-gated sodium channel (VGSC) blocker.[7] It is a selective blocker of the inactivated state of VGSCs, preferentially inhibiting persistent sodium current.[7] It has been proposed that cenobamate additionally enhances presynaptic release of γ-aminobutyric acid (GABA), thereby increasing inhibitory GABAergic neurotransmission.[7]
History
The safety and efficacy of cenobamate to treat partial-onset seizures was established in two randomized, double-blind, placebo-controlled studies that enrolled 655 adults. In these studies, patients had partial-onset seizures with or without secondary generalization for an average of approximately 24 years and median seizure frequency of 8.5 seizures per 28 days during an 8-week baseline period. During the trials, doses of 100, 200, and 400 milligrams (mg) daily reduced the percent of seizures per 28 days compared with the placebo group. The recommended maintenance dose, following a titration (medication adjustment) period, is 200 mg daily; however, some patients may need an additional titration to 400 mg daily, the maximum recommended dose, based on their clinical response and tolerability.[3]
Society and culture
Legal status
The U.S. Food and Drug Administration (FDA) approved cenobamate in November 2019, and granted the application for Xcopri to SK Life Science Inc.[3][4][5][8]
On 28 January 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Ontozry, intended for adults with epilepsy whose disease is not adequately controlled despite a history of treatment with at least two anti-epileptic medicinal products.[9] The applicant for this medicinal product is Arvelle Therapeutics Netherlands B.V.[9]
References
- "Xcopri Titration Pack- cenobamate kit Xcopri- cenobamate tablet, film coated Xcopri Maintenance Pack- cenobamate kit". DailyMed. Retrieved 1 February 2021.
- "Schedules of Controlled Substances: Placement of Cenobamate in Schedule V". Federal Register. 10 March 2020.
- "FDA approves new treatment for adults with partial-onset seizures". U.S. Food and Drug Administration (FDA) (Press release). 21 November 2019. Archived from the original on 22 November 2019. Retrieved 21 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Drug Trials Snapshots: Xcopri". U.S. Food and Drug Administration (FDA). 3 December 2019. Archived from the original on 19 December 2019. Retrieved 18 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Drug Approval Package: Xcopri". U.S. Food and Drug Administration (FDA). 10 December 2019. Archived from the original on 19 December 2019. Retrieved 18 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "2020 - Placement of Cenobamate in Schedule V". DEA Diversion Control Division. 10 March 2020. Retrieved 11 March 2020.
- Younus I, Reddy DS (January 2018). "A resurging boom in new drugs for epilepsy and brain disorders". Expert Review of Clinical Pharmacology. 11 (1): 27–45. doi:10.1080/17512433.2018.1386553. PMID 28956955.
- "Cenobamate FDA Approval Status". Drugs.com. 13 November 2019. Retrieved 22 November 2019.
- "Ontozry: Pending EC decision". European Medicines Agency (EMA). 29 January 2021. Retrieved 1 February 2021. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
External links
- "Cenobamate". Drug Information Portal. U.S. National Library of Medicine (NLM).