Felbamate
Felbamate (marketed under the brand name Felbatol by MedPointe) is an anticonvulsant[1] used in the treatment of epilepsy. It is used to treat partial seizures[2][3] (with and without generalization) in adults and partial and generalized seizures associated with Lennox–Gastaut syndrome in children. However, an increased risk of potentially fatal aplastic anemia and/or liver failure limit the drug's usage to severe refractory epilepsy.
 | |
| Clinical data | |
|---|---|
| Trade names | Felbatol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a606011 |
| Routes of administration | By mouth (tablets, oral suspension) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | >90% |
| Metabolism | Hepatic |
| Elimination half-life | 20–23 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.042.714 |
| Chemical and physical data | |
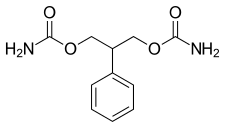
| Formula | C11H14N2O4 |
| Molar mass | 238.24 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Mechanism of action
Felbamate has been proposed to have a unique dual mechanism of action as a positive modulator of GABAA receptors[4][5] and as a blocker of NMDA receptors, particularly isoforms containing the NR2B subunit.[6][7][8][9] Although it is clear that felbamate does cause pharmacological inhibition of NMDA receptors, the relevance of NMDA receptor blockade as a strategy for the treatment of human epilepsy has been questioned.[10] Therefore, the importance of the effects of felbamate on NMDA receptors to its therapeutic action in epilepsy is uncertain.
Approval history
United States
- August 1993. Felbamate was approved for partial seizures with and without secondary generalization in adults and for Lennox–Gastaut Syndrome, a serious form of childhood epilepsy. Over the following year 150,000 people were started on felbamate therapy and a third of these became established.
- August 1, 1994. It was urgently withdrawn after 10 cases of aplastic anemia.[11] A "Dear Doctor" letter was sent to 240,000 physicians.
- September 27, 1994. Felbamate had a limited redemption in another "Dear Doctor" letter sent to 260,000 physicians. It was recommended that the drug remain available only for patients with severe epilepsy for whom the benefits outweigh the risks, and that changes be made to the product's labelling to reflect the newly recognized risk.[12] This redemption came with an additional warning since there had been 10 cases acute liver failure (4 of which were fatal). At this point, 10,000 to 12,000 people remained on the drug.
United Kingdom
- The drug is only available on a limited named-patient basis.
Indications and usage
- Adults: Monotherapy or adjunctive therapy in the treatment of partial seizures, with and without generalization.
- Children: Adjunctive therapy in the treatment of partial and generalized seizures associated with Lennox-Gastaut syndrome.
Dosing
Felbamate is available in tablets (400 mg and 600 mg) and as a peach-coloured oral suspension (600 mg/5 mL).
- Adults (≥ 14 years): begin with 1,200 mg daily given every 6 to 8 hours
- Children (2–14 years): 15 to 45 mg per kg per day given every 6 to 8 hours
Side effects
Adverse reactions include decreased appetite, vomiting, insomnia, nausea, dizziness, somnolence, and headache. Many patients report increased alertness with the drug. Two rare but very serious effects include aplastic anemia and serious liver damage. The risk of aplastic anemia is between 1:3,600 and 1:5,000, of which 30% of cases are fatal. The risk of liver damage is between 1:24,000 to 1:34,000, of which 40% of cases are fatal.
Drug interactions
Felbamate is an inhibitor of CYP2C19 - an enzyme involved in the metabolism of several commonly used medications.[13] Felbamate interacts with several other AEDs, including phenytoin, valproate, and carbamazepine; dosage adjustments may be necessary to avoid adverse effects. Concomitant administration of felbamate and carbamazepine decreases blood levels of both drugs, while increasing the level of carbamazepine-10,11 epoxide, the active metabolite of carbamazepine.[14]
History
Felbamate was discovered by Frank Berger at Wallace Laboratories.[15]
References
- Rho JM, Donevan SD, Rogawski MA (March 1997). "Barbiturate-Like Actions of the Propanediol Dicarbamates Felbamate and Meprobamate". J. Pharmacol. Exp. Ther. 280 (3): 1383–91. PMID 9067327.
- Leppik IE, Dreifuss FE, Pledger GW, et al. (November 1991). "Felbamate for Partial Seizures: Results of a Controlled Clinical Trial". Neurology. 41 (11): 1785–9. doi:10.1212/wnl.41.11.1785. PMID 1944909. S2CID 25245002.
- Devinsky O, Faught RE, Wilder BJ, et al. (March 1995). "Efficacy of Felbamate Monotherapy in Patients Undergoing Presurgical Evaluation of Partial Seizures". Epilepsy Res. 20 (3): 241–6. doi:10.1016/0920-1211(94)00084-A. PMID 7796796. S2CID 21915205.
- Rho JM, Donevan SD, Rogawski MA (Feb 1994). "Mechanism of Action of the Anticonvulsant Felbamate: Opposing Effects on N-Methyl-D-aspartate and Gamma-Aminobutyric Acid A Receptors". Annals of Neurology. 35 (2): 229–34. doi:10.1002/ana.410350216. PMID 8109904. S2CID 33913077.
- Kume A, Greenfield LJ, Macdonald RL, Albin RL (June 1996). "Felbamate Inhibits [3H]t-Butylbicycloorthobenzoate (TBOB) Binding and Enhances Cl– Current at the Gamma-Aminobutyric Acid A (GABAA) Receptor". J. Pharmacol. Exp. Ther. 277 (3): 1784–92. PMID 8667250.
- Subramaniam S, Rho JM, Penix L, Donevan SD, Fielding RP, Rogawski MA (May 1995). "Felbamate Block of the N-Methyl-D-aspartate Receptor". The Journal of Pharmacology and Experimental Therapeutics. 273 (2): 878–86. PMID 7752093.
- Kleckner NW, Glazewski JC, Chen CC, Moscrip TD (May 1999). "Subtype-Selective Antagonism of N-Methyl-D-aspartate Receptors by Felbamate: Insights into the Mechanism of Action". The Journal of Pharmacology and Experimental Therapeutics. 289 (2): 886–894. PMID 10215667.
- Harty TP, Rogawski MA (March 2000). "Felbamate Block of Recombinant N-Methyl-D-aspartate Receptors: Selectivity for the NR2B Subunit". Epilepsy Research. 39 (1): 47–55. doi:10.1016/s0920-1211(99)00108-4. PMID 10690753. S2CID 25467576.
- Chang HR, Chung-Chin Kuo CC (March 2008). "Molecular determinants of the anticonvulsant felbamate binding site in the N-methyl-D-aspartate receptor". Journal of Medicinal Chemistry. 51 (6): 1534–45. doi:10.1021/jm0706618. PMID 18311896.
- Rogawski MA (March 2011). "Revisiting AMPA Receptors as an Antiepileptic Drug Target". Epilepsy Currents. 11 (2): 56–63. doi:10.5698/1535-7511-11.2.56. PMC 3117497. PMID 21686307.
- "www.fda.gov". Archived from the original on November 2, 2008. Retrieved 2008-11-15.
- "www.fda.gov". Archived from the original on September 29, 2007. Retrieved 2008-11-15.
- Flockhart DA (2007). "Drug Interactions: Cytochrome P450 Drug Interaction Table". Indiana University School of Medicine. Retrieved on December 25, 2008.
- Curry WJ, Kulling DL (February 1998). "Newer Antiepileptic Drugs: Gabapentin, Lamotrigine, Felbamate, Topiramate and Fosphenytoin". Am Fam Physician. 57 (3): 513–20. PMID 9475899.
- "Frank Berger". Daily Telegraph. 2008-04-07. ISSN 0307-1235. Retrieved 2018-09-22.
External links
- Felbatol: Prescribing Information
- RxList: Felbamate contains extensive information including the patient warning and a sample consent form.
- Hard Choices with Felbamate
- Newer Antiepileptic Drugs: Gabapentin, Lamotrigine, Felbamate, Topiramate and Fosphenytoin
- MedPonte Pharmaceuticals