Diethyl sulfide
Diethyl sulfide is an organosulfur compound with the chemical formula (C
2H
5)
2S. It is a colorless, malodorous liquid. Although a common thioether, it has few applications.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(Ethylsulfanyl)ethane | |
| Other names
1,1-thiobisethane, diethyl thioether, ethyl sulfide, thioethyl ether | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.934 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H10S | |
| Molar mass | 90.19 |
| Appearance | Clear liquid |
| Density | 0.837 g/cm3 |
| Melting point | −103.8 °C (−154.8 °F; 169.3 K) |
| Boiling point | 92 °C (198 °F; 365 K) |
| insoluble | |
| Solubility in ethanol | miscible |
| Solubility in diethyl ether | miscible |
| −67.9·10−6 cm3/mol | |
Refractive index (nD) |
1.44233 |
| Hazards | |
| Main hazards | Skin and eye irritant. Highly flammable liquid and vapor |
| Safety data sheet | External MSDS |
| R-phrases (outdated) | R11 R65 |
| S-phrases (outdated) | (S2) S9 S16 S51 S62 |
| NFPA 704 (fire diamond) | |
| Flash point | −10 °C (14 °F; 263 K) |
| Related compounds | |
Related thioethers |
dimethyl sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
Diethyl sulfide is a by-product of the commercial production of ethanethiol, which is prepared by the reaction of ethylene with hydrogen sulfide over an alumina-based catalyst. The amount of diethyl sulfide produced can be controlled by varying the ratio of hydrogen sulfide to ethylene.
Occurrence
Diethyl sulfide has been found to be a constituent of the odor of durian fruit[1] and as a constituent found in volatiles from potatoes.[2]
Reactions
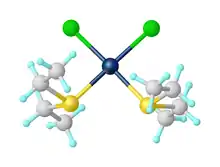
Diethyl sulfide is a Lewis base, classified as a soft ligand (see also ECW model).Its relative donor strength toward a series of acids, versus other Lewis bases, can be illustrated by C-B plots.[3][4] A typical complex is cis-PtCl2(SEt2)2.
References
- Baldry, Jane; J. Dougan; G. E. Howard (1972). "Volatile Flavouring Constituents of Durian". Phytochemistry. 11 (6): 2081–2084. doi:10.1016/s0031-9422(00)90176-6.
- Gumbmann, M. R.; H. K. Burr (1964). "Food Flavors and Odors, Volatile Sulfur Compounds in Potatoes". Journal of Agricultural and Food Chemistry. 12 (5): 404–408. doi:10.1021/jf60135a004.
- Laurence, C. and Gal, J-F. Lewis Basicity and Affinity Scales, Data and Measurement, (Wiley 2010) pp 50-51 IBSN 978-0-470-74957-9
- Cramer, R. E.; Bopp, T. T. (1977). "Graphical display of the enthalpies of adduct formation for Lewis acids and bases". Journal of Chemical Education. 54: 612–613. doi:10.1021/ed054p612. The plots shown in this paper used older parameters. Improved E&C parameters are listed in ECW model.
- C.Hansson (2007). "cis-Dichloridobis(diethyl sulfide-κS)platinum(II) at 295 and 150 K". Acta Crystallographica Section C. 63 (Pt 8): m361-3. doi:10.1107/S0108270107030417. PMID 17675684.

