Dimensional analysis
In engineering and science, dimensional analysis is the analysis of the relationships between different physical quantities by identifying their base quantities (such as length, mass, time, and electric charge) and units of measure (such as miles vs. kilometres, or pounds vs. kilograms) and tracking these dimensions as calculations or comparisons are performed. The conversion of units from one dimensional unit to another is often easier within the metric or SI system than in others, due to the regular 10-base in all units. Dimensional analysis, or more specifically the factor-label method, also known as the unit-factor method, is a widely used technique for such conversions using the rules of algebra.[1][2][3]
Commensurable physical quantities are of the same kind and have the same dimension, and can be directly compared to each other, even if they are originally expressed in differing units of measure, e.g. yards and metres, pounds(mass) and kilograms, seconds and years. Incommensurable physical quantities are of different kinds and have different dimensions, and can not be directly compared to each other, no matter what units they are originally expressed in, e.g. meters and kilograms, seconds and kilograms, meters and seconds. For example, asking whether a kilogram is larger than an hour is meaningless.
Any physically meaningful equation, or inequality, must have the same dimensions on its left and right sides, a property known as dimensional homogeneity. Checking for dimensional homogeneity is a common application of dimensional analysis, serving as a plausibility check on derived equations and computations. It also serves as a guide and constraint in deriving equations that may describe a physical system in the absence of a more rigorous derivation.
The concept of physical dimension, and of dimensional analysis, was introduced by Joseph Fourier in 1822.[4]
Concrete numbers and base units
Many parameters and measurements in the physical sciences and engineering are expressed as a concrete number—a numerical quantity and a corresponding dimensional unit. Often a quantity is expressed in terms of several other quantities; for example, speed is a combination of length and time, e.g. 60 kilometres per hour or 1.4 kilometres per second. Compound relations with "per" are expressed with division, e.g. 60 km/1 h. Other relations can involve multiplication (often shown with a centered dot or juxtaposition), powers (like m2 for square metres), or combinations thereof.
A set of base units for a system of measurement is a conventionally chosen set of units, none of which can be expressed as a combination of the others and in terms of which all the remaining units of the system can be expressed.[5] For example, units for length and time are normally chosen as base units. Units for volume, however, can be factored into the base units of length (m3), thus they are considered derived or compound units.
Sometimes the names of units obscure the fact that they are derived units. For example, a newton (N) is a unit of force, which has units of mass (kg) times units of acceleration (m⋅s−2). The newton is defined as 1 N = 1 kg⋅m⋅s−2.
Percentages and derivatives
Percentages are dimensionless quantities, since they are ratios of two quantities with the same dimensions. In other words, the % sign can be read as "hundredths", since 1% = 1/100.
Taking a derivative with respect to a quantity adds the dimension of the variable one is differentiating with respect to, in the denominator. Thus:
- position (x) has the dimension L (length);
- derivative of position with respect to time (dx/dt, velocity) has dimension LT−1—length from position, time due to the gradient;
- the second derivative (d2x/dt2 = d(dx/dt) / dt, acceleration) has dimension LT−2.
In economics, one distinguishes between stocks and flows: a stock has units of "units" (say, widgets or dollars), while a flow is a derivative of a stock, and has units of "units/time" (say, dollars/year).
In some contexts, dimensional quantities are expressed as dimensionless quantities or percentages by omitting some dimensions. For example, debt-to-GDP ratios are generally expressed as percentages: total debt outstanding (dimension of currency) divided by annual GDP (dimension of currency)—but one may argue that, in comparing a stock to a flow, annual GDP should have dimensions of currency/time (dollars/year, for instance) and thus Debt-to-GDP should have units of years, which indicates that Debt-to-GDP is the number of years needed for a constant GDP to pay the debt, if all GDP is spent on the debt and the debt is otherwise unchanged.
Conversion factor
In dimensional analysis, a ratio which converts one unit of measure into another without changing the quantity is called a conversion factor. For example, kPa and bar are both units of pressure, and 100 kPa = 1 bar. The rules of algebra allow both sides of an equation to be divided by the same expression, so this is equivalent to 100 kPa / 1 bar = 1. Since any quantity can be multiplied by 1 without changing it, the expression "100 kPa / 1 bar" can be used to convert from bars to kPa by multiplying it with the quantity to be converted, including units. For example, 5 bar × 100 kPa / 1 bar = 500 kPa because 5 × 100 / 1 = 500, and bar/bar cancels out, so 5 bar = 500 kPa.
Dimensional homogeneity
The most basic rule of dimensional analysis is that of dimensional homogeneity.[6]
- Only commensurable quantities (physical quantities having the same dimension) may be compared, equated, added, or subtracted.
However, the dimensions form an abelian group under multiplication, so:
- One may take ratios of incommensurable quantities (quantities with different dimensions), and multiply or divide them.
For example, it makes no sense to ask whether 1 hour is more, the same, or less than 1 kilometre, as these have different dimensions, nor to add 1 hour to 1 kilometre. However, it makes perfect sense to ask whether 1 mile is more, the same, or less than 1 kilometre being the same dimension of physical quantity even though the units are different. On the other hand, if an object travels 100 km in 2 hours, one may divide these and conclude that the object's average speed was 50 km/h.
The rule implies that in a physically meaningful expression only quantities of the same dimension can be added, subtracted, or compared. For example, if mman, mrat and Lman denote, respectively, the mass of some man, the mass of a rat and the length of that man, the dimensionally homogeneous expression mman + mrat is meaningful, but the heterogeneous expression mman + Lman is meaningless. However, mman/L2man is fine. Thus, dimensional analysis may be used as a sanity check of physical equations: the two sides of any equation must be commensurable or have the same dimensions.
This has the implication that most mathematical functions, particularly the transcendental functions, must have a dimensionless quantity, a pure number, as the argument and must return a dimensionless number as a result. This is clear because many transcendental functions can be expressed as an infinite power series with dimensionless coefficients.
All powers of x must have the same dimension for the terms to be commensurable. But if x is not dimensionless, then the different powers of x will have different, incommensurable dimensions. However, power functions including root functions may have a dimensional argument and will return a result having dimension that is the same power applied to the argument dimension. This is because power functions and root functions are, loosely, just an expression of multiplication of quantities.
Even when two physical quantities have identical dimensions, it may nevertheless be meaningless to compare or add them. For example, although torque and energy share the dimension L2MT−2, they are fundamentally different physical quantities.
To compare, add, or subtract quantities with the same dimensions but expressed in different units, the standard procedure is first to convert them all to the same units. For example, to compare 32 metres with 35 yards, use 1 yard = 0.9144 m to convert 35 yards to 32.004 m.
A related principle is that any physical law that accurately describes the real world must be independent of the units used to measure the physical variables.[7] For example, Newton's laws of motion must hold true whether distance is measured in miles or kilometres. This principle gives rise to the form that conversion factors must take between units that measure the same dimension: multiplication by a simple constant. It also ensures equivalence; for example, if two buildings are the same height in feet, then they must be the same height in metres.
The factor-label method for converting units
The factor-label method is the sequential application of conversion factors expressed as fractions and arranged so that any dimensional unit appearing in both the numerator and denominator of any of the fractions can be cancelled out until only the desired set of dimensional units is obtained. For example, 10 miles per hour can be converted to meters per second by using a sequence of conversion factors as shown below:
Each conversion factor is chosen based on the relationship between one of the original units and one of the desired units (or some intermediary unit), before being re-arranged to create a factor that cancels out the original unit. For example, as "mile" is the numerator in the original fraction and , "mile" will need to be the denominator in the conversion factor. Dividing both sides of the equation by 1 mile yields , which when simplified results in the dimensionless . Multiplying any quantity (physical quantity or not) by the dimensionless 1 does not change that quantity. Once this and the conversion factor for seconds per hour have been multiplied by the original fraction to cancel out the units mile and hour, 10 miles per hour converts to 4.4704 meters per second.
As a more complex example, the concentration of nitrogen oxides (i.e., ) in the flue gas from an industrial furnace can be converted to a mass flow rate expressed in grams per hour (i.e., g/h) of by using the following information as shown below:
- NOx concentration
- = 10 parts per million by volume = 10 ppmv = 10 volumes/106 volumes
- NOx molar mass
- = 46 kg/kmol = 46 g/mol
- Flow rate of flue gas
- = 20 cubic meters per minute = 20 m3/min
- The flue gas exits the furnace at 0 °C temperature and 101.325 kPa absolute pressure.
- The molar volume of a gas at 0 °C temperature and 101.325 kPa is 22.414 m3/kmol.
After canceling out any dimensional units that appear both in the numerators and denominators of the fractions in the above equation, the NOx concentration of 10 ppmv converts to mass flow rate of 24.63 grams per hour.
Checking equations that involve dimensions
The factor-label method can also be used on any mathematical equation to check whether or not the dimensional units on the left hand side of the equation are the same as the dimensional units on the right hand side of the equation. Having the same units on both sides of an equation does not ensure that the equation is correct, but having different units on the two sides (when expressed in terms of base units) of an equation implies that the equation is wrong.
For example, check the Universal Gas Law equation of PV = nRT, when:
- the pressure P is in pascals (Pa)
- the volume V is in cubic meters (m3)
- the amount of substance n is in moles (mol)
- the universal gas law constant R is 8.3145 Pa⋅m3/(mol⋅K)
- the temperature T is in kelvins (K)
As can be seen, when the dimensional units appearing in the numerator and denominator of the equation's right hand side are cancelled out, both sides of the equation have the same dimensional units. Dimensional analysis can be used as a tool to construct equations that relate non-associated physico-chemical properties. The equations may reveal hitherto unknown or overlooked properties of matter, in the form of left-over dimensions — dimensional adjusters — that can then be assigned physical significance. It is important to point out that such ‘mathematical manipulation’ is neither without prior precedent, nor without considerable scientific significance. Indeed, the Planck's constant, a fundamental constant of the universe, was ‘discovered’ as a purely mathematical abstraction or representation that built on the Rayleigh-Jeans Equation for preventing the ultraviolet catastrophe. It was assigned and ascended to its quantum physical significance either in tandem or post mathematical dimensional adjustment – not earlier.
Limitations
The factor-label method can convert only unit quantities for which the units are in a linear relationship intersecting at 0. (Ratio scale in Stevens's typology) Most units fit this paradigm. An example for which it cannot be used is the conversion between degrees Celsius and kelvins (or degrees Fahrenheit). Between degrees Celsius and kelvins, there is a constant difference rather than a constant ratio, while between degrees Celsius and degrees Fahrenheit there is neither a constant difference nor a constant ratio. There is, however, an affine transform (, rather than a linear transform ) between them.
For example, the freezing point of water is 0 °C and 32 °F, and a 5 °C change is the same as a 9 °F change. Thus, to convert from units of Fahrenheit to units of Celsius, one subtracts 32 °F (the offset from the point of reference), divides by 9 °F and multiplies by 5 °C (scales by the ratio of units), and adds 0 °C (the offset from the point of reference). Reversing this yields the formula for obtaining a quantity in units of Celsius from units of Fahrenheit; one could have started with the equivalence between 100 °C and 212 °F, though this would yield the same formula at the end.
Hence, to convert the numerical quantity value of a temperature T[F] in degrees Fahrenheit to a numerical quantity value T[C] in degrees Celsius, this formula may be used:
- T[C] = (T[F] − 32) × 5/9.
To convert T[C] in degrees Celsius to T[F] in degrees Fahrenheit, this formula may be used:
- T[F] = (T[C] × 9/5) + 32.
Applications
Dimensional analysis is most often used in physics and chemistry – and in the mathematics thereof – but finds some applications outside of those fields as well.
Mathematics
A simple application of dimensional analysis to mathematics is in computing the form of the volume of an n-ball (the solid ball in n dimensions), or the area of its surface, the n-sphere: being an n-dimensional figure, the volume scales as while the surface area, being -dimensional, scales as Thus the volume of the n-ball in terms of the radius is for some constant Determining the constant takes more involved mathematics, but the form can be deduced and checked by dimensional analysis alone.
Finance, economics, and accounting
In finance, economics, and accounting, dimensional analysis is most commonly referred to in terms of the distinction between stocks and flows. More generally, dimensional analysis is used in interpreting various financial ratios, economics ratios, and accounting ratios.
- For example, the P/E ratio has dimensions of time (units of years), and can be interpreted as "years of earnings to earn the price paid".
- In economics, debt-to-GDP ratio also has units of years (debt has units of currency, GDP has units of currency/year).
- In financial analysis, some bond duration types also have dimension of time (unit of years) and can be interpreted as ”years to balance point between interest payments and nominal repayment”.
- Velocity of money has units of 1/years (GDP/money supply has units of currency/year over currency): how often a unit of currency circulates per year.
- Interest rates are often expressed as a percentage, but more properly percent per annum, which has dimensions of 1/years.
Fluid mechanics
In fluid mechanics, dimensional analysis is performed in order to obtain dimensionless pi terms or groups. According to the principles of dimensional analysis, any prototype can be described by a series of these terms or groups that describe the behaviour of the system. Using suitable pi terms or groups, it is possible to develop a similar set of pi terms for a model that has the same dimensional relationships.[8] In other words, pi terms provide a shortcut to developing a model representing a certain prototype. Common dimensionless groups in fluid mechanics include:
- Reynolds number (Re), generally important in all types of fluid problems:
- .
- Froude number (Fr), modeling flow with a free surface:
- Euler number (Eu), used in problems in which pressure is of interest:
- Mach number (Ma), important in high speed flows where the velocity approaches or exceeds the local speed of sound:
- where: c is the local speed of sound.
History
The origins of dimensional analysis have been disputed by historians.[9][10]
The first written application of dimensional analysis has been credited to an article of François Daviet at the Turin Academy of Science. Daviet had the master Lagrange as teacher. His fundamental works are contained in acta of the Academy dated 1799.[10]
This led to the conclusion that meaningful laws must be homogeneous equations in their various units of measurement, a result which was eventually later formalized in the Buckingham π theorem. Simeon Poisson also treated the same problem of the parallelogram law by Daviet, in his treatise of 1811 and 1833 (vol I, p.39).[11] In the second edition of 1833, Poisson explicitly introduces the term dimension instead of the Daviet homogeneity.
In 1822, the important Napoleonic scientist Joseph Fourier made the first credited important contributions[12] based on the idea that physical laws like F = ma should be independent of the units employed to measure the physical variables.
Maxwell played a major role in establishing modern use of dimensional analysis by distinguishing mass, length, and time as fundamental units, while referring to other units as derived.[13] Although Maxwell defined length, time and mass to be "the three fundamental units", he also noted that gravitational mass can be derived from length and time by assuming a form of Newton's law of universal gravitation in which the gravitational constant G is taken as unity, thereby defining M = L3T−2.[14] By assuming a form of Coulomb's law in which Coulomb's constant ke is taken as unity, Maxwell then determined that the dimensions of an electrostatic unit of charge were Q = L3/2M1/2T−1,[15] which, after substituting his M = L3T−2 equation for mass, results in charge having the same dimensions as mass, viz. Q = L3T−2.
Dimensional analysis is also used to derive relationships between the physical quantities that are involved in a particular phenomenon that one wishes to understand and characterize. It was used for the first time (Pesic 2005) in this way in 1872 by Lord Rayleigh, who was trying to understand why the sky is blue. Rayleigh first published the technique in his 1877 book The Theory of Sound.[16]
The original meaning of the word dimension, in Fourier's Theorie de la Chaleur, was the numerical value of the exponents of the base units. For example, acceleration was considered to have the dimension 1 with respect to the unit of length, and the dimension −2 with respect to the unit of time.[17] This was slightly changed by Maxwell, who said the dimensions of acceleration are LT−2, instead of just the exponents.[18]
Mathematical formulation
The Buckingham π theorem describes how every physically meaningful equation involving n variables can be equivalently rewritten as an equation of n − m dimensionless parameters, where m is the rank of the dimensional matrix. Furthermore, and most importantly, it provides a method for computing these dimensionless parameters from the given variables.
A dimensional equation can have the dimensions reduced or eliminated through nondimensionalization, which begins with dimensional analysis, and involves scaling quantities by characteristic units of a system or natural units of nature. This gives insight into the fundamental properties of the system, as illustrated in the examples below.
Definition
The dimension of a physical quantity can be expressed as a product of the basic physical dimensions such as length, mass and time, each raised to a rational power. The dimension of a physical quantity is more fundamental than some scale unit used to express the amount of that physical quantity. For example, mass is a dimension, while the kilogram is a particular scale unit chosen to express a quantity of mass. Except for natural units, the choice of scale is cultural and arbitrary.
There are many possible choices of basic physical dimensions. The SI standard recommends the usage of the following dimensions and corresponding symbols: length (L), mass (M), time (T), electric current (I), absolute temperature (Θ), amount of substance (N) and luminous intensity (J). The symbols are by convention usually written in roman sans serif typeface.[19] Mathematically, the dimension of the quantity Q is given by
where a, b, c, d, e, f, g are the dimensional exponents. Other physical quantities could be defined as the base quantities, as long as they form a linearly independent basis. For instance, one could replace the dimension of electric current (I) of the SI basis with a dimension of electric charge (Q), since Q = IT.
As examples, the dimension of the physical quantity speed v is
and the dimension of the physical quantity force F is
The unit chosen to express a physical quantity and its dimension are related, but not identical concepts. The units of a physical quantity are defined by convention and related to some standard; e.g., length may have units of metres, feet, inches, miles or micrometres; but any length always has a dimension of L, no matter what units of length are chosen to express it. Two different units of the same physical quantity have conversion factors that relate them. For example, 1 in = 2.54 cm; in this case (2.54 cm/in) is the conversion factor, which is itself dimensionless. Therefore, multiplying by that conversion factor does not change the dimensions of a physical quantity.
There are also physicists that have cast doubt on the very existence of incompatible fundamental dimensions of physical quantity,[20] although this does not invalidate the usefulness of dimensional analysis.
Mathematical properties
The dimensions that can be formed from a given collection of basic physical dimensions, such as M, L, and T, form an abelian group: The identity is written as 1; L0 = 1, and the inverse to L is 1/L or L−1. L raised to any rational power p is a member of the group, having an inverse of L−p or 1/Lp. The operation of the group is multiplication, having the usual rules for handling exponents (Ln × Lm = Ln+m).
This group can be described as a vector space over the rational numbers, with for example dimensional symbol MiLjTk corresponding to the vector (i, j, k). When physical measured quantities (be they like-dimensioned or unlike-dimensioned) are multiplied or divided by one other, their dimensional units are likewise multiplied or divided; this corresponds to addition or subtraction in the vector space. When measurable quantities are raised to a rational power, the same is done to the dimensional symbols attached to those quantities; this corresponds to scalar multiplication in the vector space.
A basis for such a vector space of dimensional symbols is called a set of base quantities, and all other vectors are called derived units. As in any vector space, one may choose different bases, which yields different systems of units (e.g., choosing whether the unit for charge is derived from the unit for current, or vice versa).
The group identity 1, the dimension of dimensionless quantities, corresponds to the origin in this vector space.
The set of units of the physical quantities involved in a problem correspond to a set of vectors (or a matrix). The nullity describes some number (e.g., m) of ways in which these vectors can be combined to produce a zero vector. These correspond to producing (from the measurements) a number of dimensionless quantities, {π1, ..., πm}. (In fact these ways completely span the null subspace of another different space, of powers of the measurements.) Every possible way of multiplying (and exponentiating) together the measured quantities to produce something with the same units as some derived quantity X can be expressed in the general form
Consequently, every possible commensurate equation for the physics of the system can be rewritten in the form
Knowing this restriction can be a powerful tool for obtaining new insight into the system.
Mechanics
The dimension of physical quantities of interest in mechanics can be expressed in terms of base dimensions M, L, and T – these form a 3-dimensional vector space. This is not the only valid choice of base dimensions, but it is the one most commonly used. For example, one might choose force, length and mass as the base dimensions (as some have done), with associated dimensions F, L, M; this corresponds to a different basis, and one may convert between these representations by a change of basis. The choice of the base set of dimensions is thus a convention, with the benefit of increased utility and familiarity. The choice of base dimensions is not entirely arbitrary, because they must form a basis: they must span the space, and be linearly independent.
For example, F, L, M form a set of fundamental dimensions because they form a basis that is equivalent to M, L, T: the former can be expressed as [F = ML/T2], L, M, while the latter can be expressed as M, L, [T = (ML/F)1/2].
On the other hand, length, velocity and time (L, V, T) do not form a set of base dimensions for mechanics, for two reasons:
- There is no way to obtain mass – or anything derived from it, such as force – without introducing another base dimension (thus, they do not span the space).
- Velocity, being expressible in terms of length and time (V = L/T), is redundant (the set is not linearly independent).
Other fields of physics and chemistry
Depending on the field of physics, it may be advantageous to choose one or another extended set of dimensional symbols. In electromagnetism, for example, it may be useful to use dimensions of M, L, T, and Q, where Q represents the dimension of electric charge. In thermodynamics, the base set of dimensions is often extended to include a dimension for temperature, Θ. In chemistry, the amount of substance (the number of molecules divided by the Avogadro constant, ≈ 6.02×1023 mol−1) is defined as a base dimension, N, as well. In the interaction of relativistic plasma with strong laser pulses, a dimensionless relativistic similarity parameter, connected with the symmetry properties of the collisionless Vlasov equation, is constructed from the plasma-, electron- and critical-densities in addition to the electromagnetic vector potential. The choice of the dimensions or even the number of dimensions to be used in different fields of physics is to some extent arbitrary, but consistency in use and ease of communications are common and necessary features.
Polynomials and transcendental functions
Scalar arguments to transcendental functions such as exponential, trigonometric and logarithmic functions, or to inhomogeneous polynomials, must be dimensionless quantities. (Note: this requirement is somewhat relaxed in Siano's orientational analysis described below, in which the square of certain dimensioned quantities are dimensionless.)
While most mathematical identities about dimensionless numbers translate in a straightforward manner to dimensional quantities, care must be taken with logarithms of ratios: the identity log(a/b) = log a − log b, where the logarithm is taken in any base, holds for dimensionless numbers a and b, but it does not hold if a and b are dimensional, because in this case the left-hand side is well-defined but the right-hand side is not.
Similarly, while one can evaluate monomials (xn) of dimensional quantities, one cannot evaluate polynomials of mixed degree with dimensionless coefficients on dimensional quantities: for x2, the expression (3 m)2 = 9 m2 makes sense (as an area), while for x2 + x, the expression (3 m)2 + 3 m = 9 m2 + 3 m does not make sense.
However, polynomials of mixed degree can make sense if the coefficients are suitably chosen physical quantities that are not dimensionless. For example,
This is the height to which an object rises in time t if the acceleration of gravity is 9.8 meter per second per second and the initial upward speed is 500 meter per second. It is not necessary for t to be in seconds. For example, suppose t = 0.01 minutes. Then the first term would be
Incorporating units
The value of a dimensional physical quantity Z is written as the product of a unit [Z] within the dimension and a dimensionless numerical factor, n.[21]
When like-dimensioned quantities are added or subtracted or compared, it is convenient to express them in consistent units so that the numerical values of these quantities may be directly added or subtracted. But, in concept, there is no problem adding quantities of the same dimension expressed in different units. For example, 1 meter added to 1 foot is a length, but one cannot derive that length by simply adding 1 and 1. A conversion factor, which is a ratio of like-dimensioned quantities and is equal to the dimensionless unity, is needed:
- is identical to
The factor is identical to the dimensionless 1, so multiplying by this conversion factor changes nothing. Then when adding two quantities of like dimension, but expressed in different units, the appropriate conversion factor, which is essentially the dimensionless 1, is used to convert the quantities to identical units so that their numerical values can be added or subtracted.
Only in this manner is it meaningful to speak of adding like-dimensioned quantities of differing units.
Position vs displacement
Some discussions of dimensional analysis implicitly describe all quantities as mathematical vectors. (In mathematics scalars are considered a special case of vectors; vectors can be added to or subtracted from other vectors, and, inter alia, multiplied or divided by scalars. If a vector is used to define a position, this assumes an implicit point of reference: an origin. While this is useful and often perfectly adequate, allowing many important errors to be caught, it can fail to model certain aspects of physics. A more rigorous approach requires distinguishing between position and displacement (or moment in time versus duration, or absolute temperature versus temperature change).
Consider points on a line, each with a position with respect to a given origin, and distances among them. Positions and displacements all have units of length, but their meaning is not interchangeable:
- adding two displacements should yield a new displacement (walking ten paces then twenty paces gets you thirty paces forward),
- adding a displacement to a position should yield a new position (walking one block down the street from an intersection gets you to the next intersection),
- subtracting two positions should yield a displacement,
- but one may not add two positions.
This illustrates the subtle distinction between affine quantities (ones modeled by an affine space, such as position) and vector quantities (ones modeled by a vector space, such as displacement).
- Vector quantities may be added to each other, yielding a new vector quantity, and a vector quantity may be added to a suitable affine quantity (a vector space acts on an affine space), yielding a new affine quantity.
- Affine quantities cannot be added, but may be subtracted, yielding relative quantities which are vectors, and these relative differences may then be added to each other or to an affine quantity.
Properly then, positions have dimension of affine length, while displacements have dimension of vector length. To assign a number to an affine unit, one must not only choose a unit of measurement, but also a point of reference, while to assign a number to a vector unit only requires a unit of measurement.
Thus some physical quantities are better modeled by vectorial quantities while others tend to require affine representation, and the distinction is reflected in their dimensional analysis.
This distinction is particularly important in the case of temperature, for which the numeric value of absolute zero is not the origin 0 in some scales. For absolute zero,
- −273.15 °C ≘ 0 K = 0 °R ≘ −459.67 °F,
where the symbol ≘ means corresponds to, since although these values on the respective temperature scales correspond, they represent distinct quantities in the same way that the distances from distinct starting points to the same end point are distinct quantities, and cannot in general be equated.
For temperature differences,
- 1 K = 1 °C ≠ 1 °F = 1 °R.
(Here °R refers to the Rankine scale, not the Réaumur scale). Unit conversion for temperature differences is simply a matter of multiplying by, e.g., 1 °F / 1 K (although the ratio is not a constant value). But because some of these scales have origins that do not correspond to absolute zero, conversion from one temperature scale to another requires accounting for that. As a result, simple dimensional analysis can lead to errors if it is ambiguous whether 1 K means the absolute temperature equal to −272.15 °C, or the temperature difference equal to 1 °C.
Orientation and frame of reference
Similar to the issue of a point of reference is the issue of orientation: a displacement in 2 or 3 dimensions is not just a length, but is a length together with a direction. (This issue does not arise in 1 dimension, or rather is equivalent to the distinction between positive and negative.) Thus, to compare or combine two dimensional quantities in a multi-dimensional space, one also needs an orientation: they need to be compared to a frame of reference.
This leads to the extensions discussed below, namely Huntley's directed dimensions and Siano's orientational analysis.
Examples
A simple example: period of a harmonic oscillator
What is the period of oscillation T of a mass m attached to an ideal linear spring with spring constant k suspended in gravity of strength g? That period is the solution for T of some dimensionless equation in the variables T, m, k, and g. The four quantities have the following dimensions: T [T]; m [M]; k [M/T2]; and g [L/T2]. From these we can form only one dimensionless product of powers of our chosen variables, = [T2 · M/T2 / M = 1], and putting for some dimensionless constant C gives the dimensionless equation sought. The dimensionless product of powers of variables is sometimes referred to as a dimensionless group of variables; here the term "group" means "collection" rather than mathematical group. They are often called dimensionless numbers as well.
Note that the variable g does not occur in the group. It is easy to see that it is impossible to form a dimensionless product of powers that combines g with k, m, and T, because g is the only quantity that involves the dimension L. This implies that in this problem the g is irrelevant. Dimensional analysis can sometimes yield strong statements about the irrelevance of some quantities in a problem, or the need for additional parameters. If we have chosen enough variables to properly describe the problem, then from this argument we can conclude that the period of the mass on the spring is independent of g: it is the same on the earth or the moon. The equation demonstrating the existence of a product of powers for our problem can be written in an entirely equivalent way: , for some dimensionless constant κ (equal to from the original dimensionless equation).
When faced with a case where dimensional analysis rejects a variable (g, here) that one intuitively expects to belong in a physical description of the situation, another possibility is that the rejected variable is in fact relevant, but that some other relevant variable has been omitted, which might combine with the rejected variable to form a dimensionless quantity. That is, however, not the case here.
When dimensional analysis yields only one dimensionless group, as here, there are no unknown functions, and the solution is said to be "complete" – although it still may involve unknown dimensionless constants, such as κ.
A more complex example: energy of a vibrating wire
Consider the case of a vibrating wire of length ℓ (L) vibrating with an amplitude A (L). The wire has a linear density ρ (M/L) and is under tension s (ML/T2), and we want to know the energy E (ML2/T2) in the wire. Let π1 and π2 be two dimensionless products of powers of the variables chosen, given by
The linear density of the wire is not involved. The two groups found can be combined into an equivalent form as an equation
where F is some unknown function, or, equivalently as
where f is some other unknown function. Here the unknown function implies that our solution is now incomplete, but dimensional analysis has given us something that may not have been obvious: the energy is proportional to the first power of the tension. Barring further analytical analysis, we might proceed to experiments to discover the form for the unknown function f. But our experiments are simpler than in the absence of dimensional analysis. We'd perform none to verify that the energy is proportional to the tension. Or perhaps we might guess that the energy is proportional to ℓ, and so infer that E = ℓs. The power of dimensional analysis as an aid to experiment and forming hypotheses becomes evident.
The power of dimensional analysis really becomes apparent when it is applied to situations, unlike those given above, that are more complicated, the set of variables involved are not apparent, and the underlying equations hopelessly complex. Consider, for example, a small pebble sitting on the bed of a river. If the river flows fast enough, it will actually raise the pebble and cause it to flow along with the water. At what critical velocity will this occur? Sorting out the guessed variables is not so easy as before. But dimensional analysis can be a powerful aid in understanding problems like this, and is usually the very first tool to be applied to complex problems where the underlying equations and constraints are poorly understood. In such cases, the answer may depend on a dimensionless number such as the Reynolds number, which may be interpreted by dimensional analysis.
A third example: demand versus capacity for a rotating disc
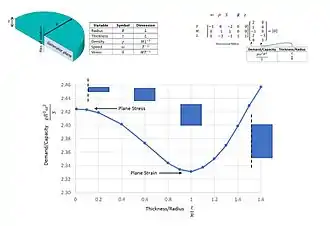
Consider the case of a thin, solid, parallel-sided rotating disc of axial thickness t (L) and radius R (L). The disc has a density ρ (M/L3), rotates at an angular velocity ω (T−1) and this leads to a stress S (ML−1T−2) in the material. There is a theoretical linear elastic solution, given by Lame, to this problem when the disc is thin relative to its radius, the faces of the disc are free to move axially, and the plane stress constitutive relations can be assumed to be valid. As the disc becomes thicker relative to the radius then the plane stress solution breaks down. If the disc is restrained axially on its free faces then a state of plane strain will occur. However, if this is not the case then the state of stress may only be determined though consideration of three-dimensional elasticity and there is no known theoretical solution for this case. An engineer might, therefore, be interested in establishing a relationship between the five variables. Dimensional analysis for this case leads to the following (5 − 3 = 2) non-dimensional groups:
- demand/capacity = ρR2ω2/S
- thickness/radius or aspect ratio = t/R
Through the use of numerical experiments using, for example, the finite element method, the nature of the relationship between the two non-dimensional groups can be obtained as shown in the figure. As this problem only involves two non-dimensional groups, the complete picture is provided in a single plot and this can be used as a design/assessment chart for rotating discs[22]
Extensions
Huntley's extension: directed dimensions and quantity of matter
Huntley (Huntley 1967) has pointed out that a dimensional analysis can become more powerful by discovering new independent dimensions in the quantities under consideration, thus increasing the rank of the dimensional matrix. He introduced two approaches to doing so:
- The magnitudes of the components of a vector are to be considered dimensionally independent. For example, rather than an undifferentiated length dimension L, we may have Lx represent dimension in the x-direction, and so forth. This requirement stems ultimately from the requirement that each component of a physically meaningful equation (scalar, vector, or tensor) must be dimensionally consistent.
- Mass as a measure of the quantity of matter is to be considered dimensionally independent from mass as a measure of inertia.
As an example of the usefulness of the first approach, suppose we wish to calculate the distance a cannonball travels when fired with a vertical velocity component and a horizontal velocity component , assuming it is fired on a flat surface. Assuming no use of directed lengths, the quantities of interest are then , , both dimensioned as LT−1, R, the distance travelled, having dimension L, and g the downward acceleration of gravity, with dimension LT−2.
With these four quantities, we may conclude that the equation for the range R may be written:
Or dimensionally
from which we may deduce that and , which leaves one exponent undetermined. This is to be expected since we have two fundamental dimensions L and T, and four parameters, with one equation.
If, however, we use directed length dimensions, then will be dimensioned as LxT−1, as LyT−1, R as Lx and g as LyT−2. The dimensional equation becomes:
and we may solve completely as , and . The increase in deductive power gained by the use of directed length dimensions is apparent.
In his second approach, Huntley holds that it is sometimes useful (e.g., in fluid mechanics and thermodynamics) to distinguish between mass as a measure of inertia (inertial mass), and mass as a measure of the quantity of matter. Quantity of matter is defined by Huntley as a quantity (a) proportional to inertial mass, but (b) not implicating inertial properties. No further restrictions are added to its definition.
For example, consider the derivation of Poiseuille's Law. We wish to find the rate of mass flow of a viscous fluid through a circular pipe. Without drawing distinctions between inertial and substantial mass we may choose as the relevant variables
- the mass flow rate with dimension MT−1
- the pressure gradient along the pipe with dimension ML−2T−2
- ρ the density with dimension ML−3
- η the dynamic fluid viscosity with dimension ML−1T−1
- r the radius of the pipe with dimension L
There are three fundamental variables so the above five equations will yield two dimensionless variables which we may take to be and and we may express the dimensional equation as
where C and a are undetermined constants. If we draw a distinction between inertial mass with dimension and quantity of matter with dimension , then mass flow rate and density will use quantity of matter as the mass parameter, while the pressure gradient and coefficient of viscosity will use inertial mass. We now have four fundamental parameters, and one dimensionless constant, so that the dimensional equation may be written:
where now only C is an undetermined constant (found to be equal to by methods outside of dimensional analysis). This equation may be solved for the mass flow rate to yield Poiseuille's law.
Huntley's recognition of quantity of matter as an independent quantity dimension is evidently successful in the problems where it is applicable, but his definition of quantity of matter is open to interpretation, as it lacks specificity beyond the two requirements (a) and (b) he postulated for it. For a given substance, the SI dimension amount of substance, with unit mole, does satisfy Huntley's two requirements as a measure of quantity of matter, and could be used as a quantity of matter in any problem of dimensional analysis where Huntley's concept is applicable.
Huntley's concept of directed length dimensions however has some serious limitations:
- It does not deal well with vector equations involving the cross product,
- nor does it handle well the use of angles as physical variables.
It also is often quite difficult to assign the L, Lx, Ly, Lz, symbols to the physical variables involved in the problem of interest. He invokes a procedure that involves the "symmetry" of the physical problem. This is often very difficult to apply reliably: It is unclear as to what parts of the problem that the notion of "symmetry" is being invoked. Is it the symmetry of the physical body that forces are acting upon, or to the points, lines or areas at which forces are being applied? What if more than one body is involved with different symmetries?
Consider the spherical bubble attached to a cylindrical tube, where one wants the flow rate of air as a function of the pressure difference in the two parts. What are the Huntley extended dimensions of the viscosity of the air contained in the connected parts? What are the extended dimensions of the pressure of the two parts? Are they the same or different? These difficulties are responsible for the limited application of Huntley's directed length dimensions to real problems.
Siano's extension: orientational analysis
Angles are, by convention, considered to be dimensionless quantities. As an example, consider again the projectile problem in which a point mass is launched from the origin (x, y) = (0, 0) at a speed v and angle θ above the x-axis, with the force of gravity directed along the negative y-axis. It is desired to find the range R, at which point the mass returns to the x-axis. Conventional analysis will yield the dimensionless variable π = R g/v2, but offers no insight into the relationship between R and θ.
Siano (1985-I, 1985-II) has suggested that the directed dimensions of Huntley be replaced by using orientational symbols 1x 1y 1z to denote vector directions, and an orientationless symbol 10. Thus, Huntley's Lx becomes L 1x with L specifying the dimension of length, and 1x specifying the orientation. Siano further shows that the orientational symbols have an algebra of their own. Along with the requirement that 1i−1 = 1i, the following multiplication table for the orientation symbols results:
Note that the orientational symbols form a group (the Klein four-group or "Viergruppe"). In this system, scalars always have the same orientation as the identity element, independent of the "symmetry of the problem". Physical quantities that are vectors have the orientation expected: a force or a velocity in the z-direction has the orientation of 1z. For angles, consider an angle θ that lies in the z-plane. Form a right triangle in the z-plane with θ being one of the acute angles. The side of the right triangle adjacent to the angle then has an orientation 1x and the side opposite has an orientation 1y. Since (using ~ to indicate orientational equivalence) tan(θ) = θ + ... ~ 1y/1x we conclude that an angle in the xy-plane must have an orientation 1y/1x = 1z, which is not unreasonable. Analogous reasoning forces the conclusion that sin(θ) has orientation 1z while cos(θ) has orientation 10. These are different, so one concludes (correctly), for example, that there are no solutions of physical equations that are of the form a cos(θ) + b sin(θ), where a and b are real scalars. Note that an expression such as is not dimensionally inconsistent since it is a special case of the sum of angles formula and should properly be written:
which for and yields . Siano distinguishes between geometric angles, which have an orientation in 3-dimensional space, and phase angles associated with time-based oscillations, which have no spatial orientation, i.e. the orientation of a phase angle is .
The assignment of orientational symbols to physical quantities and the requirement that physical equations be orientationally homogeneous can actually be used in a way that is similar to dimensional analysis to derive a little more information about acceptable solutions of physical problems. In this approach one sets up the dimensional equation and solves it as far as one can. If the lowest power of a physical variable is fractional, both sides of the solution is raised to a power such that all powers are integral. This puts it into "normal form". The orientational equation is then solved to give a more restrictive condition on the unknown powers of the orientational symbols, arriving at a solution that is more complete than the one that dimensional analysis alone gives. Often the added information is that one of the powers of a certain variable is even or odd.
As an example, for the projectile problem, using orientational symbols, θ, being in the xy-plane will thus have dimension 1z and the range of the projectile R will be of the form:
Dimensional homogeneity will now correctly yield a = −1 and b = 2, and orientational homogeneity requires that . In other words, that c must be an odd integer. In fact the required function of theta will be sin(θ)cos(θ) which is a series consisting of odd powers of θ.
It is seen that the Taylor series of sin(θ) and cos(θ) are orientationally homogeneous using the above multiplication table, while expressions like cos(θ) + sin(θ) and exp(θ) are not, and are (correctly) deemed unphysical.
Siano's orientational analysis is compatible with the conventional conception of angular quantities as being dimensionless, and within orientational analysis, the radian may still be considered a dimensionless unit. The orientational analysis of a quantity equation is carried out separately from the ordinary dimensional analysis, yielding information that supplements the dimensional analysis.
Dimensionless concepts
Constants
The dimensionless constants that arise in the results obtained, such as the C in the Poiseuille's Law problem and the in the spring problems discussed above, come from a more detailed analysis of the underlying physics and often arise from integrating some differential equation. Dimensional analysis itself has little to say about these constants, but it is useful to know that they very often have a magnitude of order unity. This observation can allow one to sometimes make "back of the envelope" calculations about the phenomenon of interest, and therefore be able to more efficiently design experiments to measure it, or to judge whether it is important, etc.
Formalisms
Paradoxically, dimensional analysis can be a useful tool even if all the parameters in the underlying theory are dimensionless, e.g., lattice models such as the Ising model can be used to study phase transitions and critical phenomena. Such models can be formulated in a purely dimensionless way. As we approach the critical point closer and closer, the distance over which the variables in the lattice model are correlated (the so-called correlation length, ) becomes larger and larger. Now, the correlation length is the relevant length scale related to critical phenomena, so one can, e.g., surmise on "dimensional grounds" that the non-analytical part of the free energy per lattice site should be where is the dimension of the lattice.
It has been argued by some physicists, e.g., M. J. Duff,[20][23] that the laws of physics are inherently dimensionless. The fact that we have assigned incompatible dimensions to Length, Time and Mass is, according to this point of view, just a matter of convention, borne out of the fact that before the advent of modern physics, there was no way to relate mass, length, and time to each other. The three independent dimensionful constants: c, ħ, and G, in the fundamental equations of physics must then be seen as mere conversion factors to convert Mass, Time and Length into each other.
Just as in the case of critical properties of lattice models, one can recover the results of dimensional analysis in the appropriate scaling limit; e.g., dimensional analysis in mechanics can be derived by reinserting the constants ħ, c, and G (but we can now consider them to be dimensionless) and demanding that a nonsingular relation between quantities exists in the limit , and . In problems involving a gravitational field the latter limit should be taken such that the field stays finite.
Dimensional equivalences
Following are tables of commonly occurring expressions in physics, related to the dimensions of energy, momentum, and force.[24][25][26]
SI units
| Energy, E
ML2T−2 |
Expression | Nomenclature |
|---|---|---|
| Mechanical | F = force, d = distance | |
| S = action, t = time, P = power | ||
| m = mass, v = velocity, p = momentum | ||
| L = angular momentum, I = moment of inertia, ω = angular velocity | ||
| Ideal gases | p = pressure, Volume, T = temperature N = amount of substance | |
| Waves | I = wave intensity, S = Poynting vector | |
| Electromagnetic | q = electric charge, ϕ = electric potential (for changes this is voltage) | |
| E = electric field, B = magnetic field, ε = permittivity, μ = permeability, V = 3d volume | ||
| p = electric dipole moment, m = magnetic moment, A = area (bounded by a current loop), I = electric current in loop |
| Momentum, p
MLT−1 |
Expression | Nomenclature |
|---|---|---|
| Mechanical | m = mass, v = velocity, F = force, t = time | |
| S = action, L = angular momentum, r = displacement | ||
| Thermal | = root mean square velocity, m = mass (of a molecule) | |
| Waves | ρ = density, V = volume, v = phase velocity | |
| Electromagnetic | A = magnetic vector potential |
| Force, F
MLT−2 |
Expression | Nomenclature |
|---|---|---|
| Mechanical | m = mass, a = acceleration | |
| Thermal | S = entropy, T = temperature, r = displacement (see entropic force) | |
| Electromagnetic | E = electric field, B = magnetic field, v = velocity, q = charge |
Natural units
If c = ħ = 1, where c is the speed of light and ħ is the reduced Planck constant, and a suitable fixed unit of energy is chosen, then all quantities of length L, mass M and time T can be expressed (dimensionally) as a power of energy E, because length, mass and time can be expressed using speed v, action S, and energy E:[26]
though speed and action are dimensionless (v = c = 1 and S = ħ = 1) – so the only remaining quantity with dimension is energy. In terms of powers of dimensions:
This is particularly useful in particle physics and high energy physics, in which case the energy unit is the electron volt (eV). Dimensional checks and estimates become very simple in this system.
However, if electric charges and currents are involved, another unit to be fixed is for electric charge, normally the electron charge e though other choices are possible.
| Quantity | p, q, r powers of energy | n power of energy | ||
|---|---|---|---|---|
| p | q | r | n | |
| Action, S | 1 | 2 | –1 | 0 |
| Speed, v | 0 | 1 | –1 | 0 |
| Mass, M | 1 | 0 | 0 | 1 |
| Length, L | 0 | 1 | 0 | –1 |
| Time, t | 0 | 0 | 1 | –1 |
| Momentum, p | 1 | 1 | –1 | 1 |
| Energy, E | 1 | 2 | –2 | 1 |
See also
- Buckingham π theorem
- Dimensionless numbers in fluid mechanics
- Fermi estimate — used to teach dimensional analysis
- Rayleigh's method of dimensional analysis
- Similitude (model) — an application of dimensional analysis
- System of measurement
Related areas of math
Programming languages
Dimensional correctness as part of type checking has been studied since 1977.[27]
Implementations for Ada[28] and C++[29] were described in 1985 and 1988.
Kennedy's 1996 thesis describes an implementation in Standard ML, [30] and later in F#.[31] There are implementations for Haskell,[32] OCaml,[33] and Rust,[34] Python,[35] and a code checker for Fortran.[36]
Griffioen's 2019 thesis extended Kennedy's Hindley–Milner type system to support Hart's matrices.[37][38]
Notes
- Goldberg, David (2006). Fundamentals of Chemistry (5th ed.). McGraw-Hill. ISBN 978-0-07-322104-5.
- Ogden, James (1999). The Handbook of Chemical Engineering. Research & Education Association. ISBN 978-0-87891-982-6.
- "Dimensional Analysis or the Factor Label Method". Mr Kent's Chemistry Page.
- Fourier, Joseph (1822), Theorie analytique de la chaleur (in French), Paris: Firmin Didot
- JCGM 200 (2012). International vocabulary of metrology – Basic and general concepts and associated terms (VIM) (PDF) (3rd ed.). Archived from the original (PDF) on 2015-09-23. Retrieved 2015-06-02.
- Cimbala, John; Çengel, Yunus (2006). "§7-2 Dimensional homogeneity". Essential of Fluid Mechanics: Fundamentals and Applications. McGraw-Hill. p. 203–. ISBN 9780073138350.
- de Jong, Frits J.; Quade, Wilhelm (1967). Dimensional analysis for economists. North Holland. p. 28.
- Waite, Lee; Fine, Jerry (2007). Applied Biofluid Mechanics. New York: McGraw-Hill. p. 260. ISBN 978-0-07-147217-3.
- Macagno, Enzo O. (1971). "Historico-critical review of dimensional analysis". Journal of the Franklin Institute. 292 (6): 391–40. doi:10.1016/0016-0032(71)90160-8.
- Martins, Roberto De A. (1981). "The origin of dimensional analysis". Journal of the Franklin Institute. 311 (5): 331–7. doi:10.1016/0016-0032(81)90475-0.
- Martins, p.403 in the Proceedings book containing his article
- Mason, Stephen Finney (1962), A history of the sciences, New York: Collier Books, p. 169, ISBN 978-0-02-093400-4
- Roche, John J (1998), The Mathematics of Measurement: A Critical History, Springer, p. 203, ISBN 978-0-387-91581-4,
Beginning apparently with Maxwell, mass, length and time began to be interpreted as having a privileged fundamental character and all other quantities as derivative, not merely with respect to measurement, but with respect to their physical status as well.
- Maxwell, James Clerk (1873), A Treatise on Electricity and Magnetism, p. 4
- Maxwell, James Clerk (1873), A Treatise on Electricity and Magnetism, Oxford, p. 45, hdl:2027/uc1.l0065867749
- Rayleigh, Baron John William Strutt (1877), The Theory of Sound, Macmillan
- Fourier (1822), p. 156.
- Maxwell, James Clerk (1873), A Treatise on Electricity and Magnetism, volume 1, p. 5
- "SI Brochure (8th edition). Section 1.3: Dimensions of quantities". BIPM. Retrieved 2013-08-08.
- Duff, M.J.; Okun, L.B.; Veneziano, G. (September 2002), "Trialogue on the number of fundamental constants", Journal of High Energy Physics, 2002 (3): 023, arXiv:physics/0110060, Bibcode:2002JHEP...03..023D, doi:10.1088/1126-6708/2002/03/023, S2CID 15806354
- For a review of the different conventions in use see: Pisanty, E (2013-09-17). "Square bracket notation for dimensions and units: usage and conventions". Physics Stack Exchange. Retrieved 2014-07-15.
- Ramsay, Angus. "Dimensional Analysis and Numerical Experiments for a Rotating Disc". Ramsay Maunder Associates. Retrieved 15 April 2017.
- Duff, M.J. (July 2004). "Comment on time-variation of fundamental constants". arXiv:hep-th/0208093v3.
- Woan, G. (2010), The Cambridge Handbook of Physics Formulas, Cambridge University Press, ISBN 978-0-521-57507-2
- Mosca, Gene; Tipler, Paul Allen (2007), Physics for Scientists and Engineers – with Modern Physics (6th ed.), San Francisco: W. H. Freeman, ISBN 978-0-7167-8964-2
- Martin, B.R.; Shaw, G.; Manchester Physics (2008), Particle Physics (2nd ed.), Wiley, ISBN 978-0-470-03294-7
- Gehani, N. (1977). "Units of measure as a data attribute". Comput. Lang. 2 (3): 93–111. doi:10.1016/0096-0551(77)90010-8.
- Gehani, N. (June 1985). "Ada's derived types and units of measure". Softw. Pract. Exper. 15 (6): 555–569. doi:10.1002/spe.4380150604. S2CID 40558757.
- Cmelik, R. F.; Gehani, N. H. (May 1988). "Dimensional analysis with C++". IEEE Software. 5 (3): 21–27. doi:10.1109/52.2021. S2CID 22450087.
- Kennedy, Andrew J. (April 1996). Programming languages and dimensions (Phd). 391. University of Cambridge. ISSN 1476-2986. UCAM-CL-TR-391.
- Kennedy, A. (2010). "Types for Units-of-Measure: Theory and Practice". In Horváth, Z.; Plasmeijer, R.; Zsók, V. (eds.). Central European Functional Programming School. CEFP 2009. Lecture Notes in Computer Science. 6299. Springer. pp. 268–305. CiteSeerX 10.1.1.174.6901. doi:10.1007/978-3-642-17685-2_8. ISBN 978-3-642-17684-5.
- Gundry, Adam (December 2015). "A typechecker plugin for units of measure: domain-specific constraint solving in GHC Haskell" (PDF). SIGPLAN Not. 50 (12): 11–22. doi:10.1145/2887747.2804305.
- Garrigue, J.; Ly, D. (2017). "Des unités dans le typeur" (PDF). 28ièmes Journées Francophones des Langaeges Applicatifs, Jan 2017, Gourette, France (in French). hal-01503084.
- Teller, David (January 2020). "Units of Measure in Rust with Refinement Types".
- Byrnes, Steve. "numericalunits (Python library)".
- "CamFort: Specify, verify, and refactor Fortran code". University of Cambridge; University of Kent. 2018.
- Hart 1995
- Griffioen, P. (2019). A unit-aware matrix language and its application in control and auditing (PDF) (Thesis). University of Amsterdam. hdl:11245.1/fd7be191-700f-4468-a329-4c8ecd9007ba.
References
- Barenblatt, G. I. (1996), Scaling, Self-Similarity, and Intermediate Asymptotics, Cambridge, UK: Cambridge University Press, ISBN 978-0-521-43522-2
- Bhaskar, R.; Nigam, Anil (1990), "Qualitative Physics Using Dimensional Analysis", Artificial Intelligence, 45 (1–2): 73–111, doi:10.1016/0004-3702(90)90038-2
- Bhaskar, R.; Nigam, Anil (1991), "Qualitative Explanations of Red Giant Formation", The Astrophysical Journal, 372: 592–6, Bibcode:1991ApJ...372..592B, doi:10.1086/170003
- Boucher; Alves (1960), "Dimensionless Numbers", Chemical Engineering Progress, 55: 55–64
- Bridgman, P. W. (1922), Dimensional Analysis, Yale University Press, ISBN 978-0-548-91029-0
- Buckingham, Edgar (1914), "On Physically Similar Systems: Illustrations of the Use of Dimensional Analysis", Physical Review, 4 (4): 345–376, Bibcode:1914PhRv....4..345B, doi:10.1103/PhysRev.4.345, hdl:10338.dmlcz/101743
- Drobot, S. (1953–1954), "On the foundations of dimensional analysis" (PDF), Studia Mathematica, 14: 84–99, doi:10.4064/sm-14-1-84-99
- Gibbings, J.C. (2011), Dimensional Analysis, Springer, ISBN 978-1-84996-316-9
- Hart, George W. (1994), "The theory of dimensioned matrices", in Lewis, John G. (ed.), Proceedings of the Fifth SIAM Conference on Applied Linear Algebra, SIAM, pp. 186–190, ISBN 978-0-89871-336-7 As postscript
- Hart, George W. (1995), Multidimensional Analysis: Algebras and Systems for Science and Engineering, Springer-Verlag, ISBN 978-0-387-94417-3
- Huntley, H. E. (1967), Dimensional Analysis, Dover, LOC 67-17978
- Klinkenberg, A. (1955), "Dimensional systems and systems of units in physics with special reference to chemical engineering: Part I. The principles according to which dimensional systems and systems of units are constructed", Chemical Engineering Science, 4 (3): 130–140, 167–177, doi:10.1016/0009-2509(55)80004-8
- Langhaar, Henry L. (1951), Dimensional Analysis and Theory of Models, Wiley, ISBN 978-0-88275-682-0
- Mendez, P.F.; Ordóñez, F. (September 2005), "Scaling Laws From Statistical Data and Dimensional Analysis", Journal of Applied Mechanics, 72 (5): 648–657, Bibcode:2005JAM....72..648M, CiteSeerX 10.1.1.422.610, doi:10.1115/1.1943434
- Moody, L. F. (1944), "Friction Factors for Pipe Flow", Transactions of the American Society of Mechanical Engineers, 66 (671)
- Murphy, N. F. (1949), "Dimensional Analysis", Bulletin of the Virginia Polytechnic Institute, 42 (6)
- Perry, J. H.; et al. (1944), "Standard System of Nomenclature for Chemical Engineering Unit Operations", Transactions of the American Institute of Chemical Engineers, 40 (251)
- Pesic, Peter (2005), Sky in a Bottle, MIT Press, pp. 227–8, ISBN 978-0-262-16234-0
- Petty, G. W. (2001), "Automated computation and consistency checking of physical dimensions and units in scientific programs", Software – Practice and Experience, 31 (11): 1067–76, doi:10.1002/spe.401, S2CID 206506776
- Porter, Alfred W. (1933), The Method of Dimensions (3rd ed.), Methuen
- J. W. Strutt (3rd Baron Rayleigh) (1915), "The Principle of Similitude", Nature, 95 (2368): 66–8, Bibcode:1915Natur..95...66R, doi:10.1038/095066c0
- Siano, Donald (1985), "Orientational Analysis – A Supplement to Dimensional Analysis – I", Journal of the Franklin Institute, 320 (6): 267–283, doi:10.1016/0016-0032(85)90031-6
- Siano, Donald (1985), "Orientational Analysis, Tensor Analysis and The Group Properties of the SI Supplementary Units – II", Journal of the Franklin Institute, 320 (6): 285–302, doi:10.1016/0016-0032(85)90032-8
- Silberberg, I. H.; McKetta, J. J. Jr. (1953), "Learning How to Use Dimensional Analysis", Petroleum Refiner, 32 (4): 5, (5): 147, (6): 101, (7): 129
- Van Driest, E. R. (March 1946), "On Dimensional Analysis and the Presentation of Data in Fluid Flow Problems", Journal of Applied Mechanics, 68 (A–34)
- Whitney, H. (1968), "The Mathematics of Physical Quantities, Parts I and II", American Mathematical Monthly, 75 (2): 115–138, 227–256, doi:10.2307/2315883, JSTOR 2315883
- Vignaux, GA (1992), Erickson, Gary J.; Neudorfer, Paul O. (eds.), Dimensional Analysis in Data Modelling, Kluwer Academic, ISBN 978-0-7923-2031-9
- Kasprzak, Wacław; Lysik, Bertold; Rybaczuk, Marek (1990), Dimensional Analysis in the Identification of Mathematical Models, World Scientific, ISBN 978-981-02-0304-7
External links
| The Wikibook Fluid Mechanics has a page on the topic of: Dimensional analysis |
| Wikimedia Commons has media related to Dimensional analysis. |
- List of dimensions for variety of physical quantities
- Unicalc Live web calculator doing units conversion by dimensional analysis
- A C++ implementation of compile-time dimensional analysis in the Boost open-source libraries
- Buckingham’s pi-theorem
- Quantity System calculator for units conversion based on dimensional approach
- Units, quantities, and fundamental constants project dimensional analysis maps
- Bowley, Roger (2009). "[ ] Dimensional Analysis". Sixty Symbols. Brady Haran for the University of Nottingham.
- Dureisseix, David (2019). An introduction to dimensional analysis (lecture). INSA Lyon.
Converting units
- Unicalc Live web calculator doing units conversion by dimensional analysis
- Math Skills Review
- U.S. EPA tutorial
- A Discussion of Units
- Short Guide to Unit Conversions
- Canceling Units Lesson
- Chapter 11: Behavior of Gases Chemistry: Concepts and Applications, Denton Independent School District
- Air Dispersion Modeling Conversions and Formulas
- www.gnu.org/software/units free program, very practical