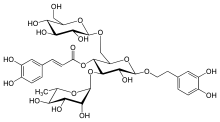
Echinacoside
Echinacoside is a natural phenol. It is a caffeic acid glycoside from the phenylpropanoid class. It is constituted from a trisaccharide consisting of two glucose and one rhamnose moieties glycosidically linked to one caffeic acid and one dihydroxyphenylethanol (hydroxytyrosol) residue at the centrally situated rhamnose.[1] This water-soluble glycoside is a distinctive secondary metabolite of Echinacea angustifolia and Echinacea pallida (to about 1%) but only occurs in trace amounts in Echinacea purpurea. It is also isolated from Cistanche spp.
 | |
| Names | |
|---|---|
| IUPAC name
[(2R,3R,4R,5R,6R)-6-[2-(3,4-dihydroxyphenyl)ethoxy]-5-hydroxy-2-[[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]-4-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-3-yl](E)-3-(3,4-dihydroxyphenyl)prop-2-enoate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.127.421 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C35H46O20 | |
| Molar mass | 786,73 g/mol |
| Melting point | 200 to 220 °C (392 to 428 °F; 473 to 493 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It was first isolated by Stoll et al. in 1950 from the roots of Echinacea angustifolia. It shows weak antibiotic activity in vitro against Staphylococcus aureus and Streptococci.[2]
References
- Bernaś, Urszula; Hajmowicz, Halina; Madura, Izabela D.; Majcher, Monika; Synoradzki, Ludwik; Zawada, Krzysztof (2010). "Tartaric acid and its acyl derivatives. Part 5. Direct synthesis of monoacyltartaric acids and novel mono(benzoyl)tartaric anhydride: unusual findings in tartaric acid acylation". Arkivoc. 2010 (11): 1–12. doi:10.3998/ark.5550190.0011.b01.
- Stoll, A.; Renz, J.; Brack, A. (1950). "Isolierung und Konstitution des Echinacosids, eines Glykosids aus den Wurzeln von Echinacea angustifolia D. C. 6. Mitteilung über antibakterielle Stoffe". Helvetica Chimica Acta. 33 (6): 1877–1893. doi:10.1002/hlca.19500330657.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.