Rosmarinic acid
Rosmarinic acid is a chemical compound found in a variety of plants. It was found to exhibit photoprotective effect against ultraviolet C (UVC) damage when examined in vitro. [2]
 | |
| Names | |
|---|---|
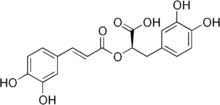
| IUPAC name
(2R)-3-(3,4-dihydroxyphenyl)-2-{[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}propanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.123.507 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H16O8 | |
| Molar mass | 360.318 g·mol−1 |
| Appearance | Red-orange powder |
| Melting point | 171 to 175 °C (340 to 347 °F; 444 to 448 K) |
| Slightly soluble | |
| Solubility in other solvents | Well soluble in most organic solvents[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
History
Rosmarinic acid was first isolated and characterized in 1958 by the Italian chemists M. L. Scarpatti and G. Oriente from rosemary (Rosmarinus officinalis).[3]
Chemistry
Chemically, rosmarinic acid is a caffeic acid ester of 3-(3,4-dihydroxyphenyl)lactic acid.
Natural occurrences
Rosmarinic acid accumulation is shown in hornworts, in the fern family Blechnaceae and in species of several orders of mono- and dicotyledonous angiosperms.[4]
It is found most notably in many Lamiaceae (dicotyledons in the order Lamiales), especially in the subfamily Nepetoideae.[5] It is found in species used commonly as culinary herbs such as Ocimum basilicum (basil), Ocimum tenuiflorum (holy basil), Melissa officinalis (lemon balm), Rosmarinus officinalis (rosemary), Origanum majorana (marjoram), Salvia officinalis (sage), thyme and peppermint.[6] It is also found in plants in the family Marantaceae (monocotyledons in the order Zingiberales)[4] such as species in the genera Maranta (Maranta leuconeura, Maranta depressa) and Thalia (Thalia geniculata).[7]
Rosmarinic acid and the derivative rosmarinic acid 3′-O-β-D-glucoside can be found in Anthoceros agrestis, a hornwort (Anthocerotophyta).[8]
Biosynthesis
The biosyntheses of rosmarinic acid uses 4-coumaroyl-CoA from the general phenylpropanoid pathway as hydroxycinnamoyl donor. The hydroxycinnamoyl acceptor substrate comes from the shikimate pathway: shikimic acid, quinic acid and 3,4-dihydroxyphenyllactic acid derived from L-tyrosine.[4] Thus, chemically, rosmarinic acid is an ester of caffeic acid with 3,4-dihydroxyphenyllactic acid, but biologically, it is formed from 4-coumaroyl-4'-hydroxyphenyllactate.[9] Rosmarinate synthase is an enzyme that uses caffeoyl-CoA and 3,4-dihydroxyphenyllactic acid to produce CoA and rosmarinate. Hydroxyphenylpyruvate reductase is also an enzyme involved in this biosynthesis.[10]
References
- "Rosmarinic acid". sigmaaldrich.com.
- Mahendra, Camille Keisha; Tan, Loh Teng-Hern; Yap, Wei Hsum; Chan, Chim Kei; Lingham, Prithvy; Pusparajah, Priyia; Htar, Thet Thet; Chuah, Lay-Hong; Goh, Bey Hing (2019). "Model of Experimentation for Photoprotective Properties of Natural Products Against Ultraviolet C (UVC) Damage: A Case Study on Rosmarinic Acid". Progress in Drug Discovery & Biomedical Science. 2. doi:10.36877/pddbs.a0000027.
- Isolamento costituzione e dell 'acido rosmarinico (dal rosmarinus off ). M. L. Scarpati, G. Oriente, Ric. Sci, 1958, volume 28, pages 2329-2333
- Evolution of rosmarinic acid biosynthesis. Petersen M, Abdullah Y, Benner J, Eberle D, Gehlen K, Hücherig S, Janiak V, Kim KH, Sander M, Weitzel C and Wolters S, Phytochemistry, Oct-Nov 2009, volume 70, issues 15-16, pages 1663-1679, doi:10.1016/j.phytochem.2009.05.010
- Distribution and taxonomic implications of some phenolics in the family Lamiaceae determined by ESR spectroscopy. J. A. Pedersen, Biochemical Systematics and Ecology, 2000, volume 28, pages 229–253
- Clifford, M.N. Chlorogenic acids and other cinnamates. Nature, occurrence and dietary burden. J. Sci. Food. Agric. (79) 362-372, 1999
- Occurrence of rosmarinic acid, chlorogenic acid and rutin in Marantaceae species. Yana Abdullah, Bernd Schneider and Maike Petersen, Phytochemistry Letters, 12 December 2008, Volume 1, Issue 4, Pages 199–203, doi:10.1016/j.phytol.2008.09.010
- Production of rosmarinic acid and a new rosmarinic acid 3′- O -β-D -glucoside in suspension cultures of the hornwort Anthoceros agrestis Paton. Katharina Vogelsang, Bernd Schneider and Maike Petersen, Planta, Volume 223, Number 2, 369-373, doi:10.1007/s00425-005-0089-8
- "MetaCyc rosmarinic acid biosynthesis I". biocyc.org.
- Two new enzymes of rosmarinic acid biosynthesis from cell cultures of Coleus blumei: hydroxyphenylpyruvate reductase and rosmarinic acid synthase. Petersen M and Alfermann AW, Z. Naturforsch. C: Biosci., 1988, volume 43, pages 501–504
